
Research Article
Austin J Anal Pharm Chem. 2019; 6(1): 1111.
Synthesis and Biological Evaluation of Some Novel Substituted 1,3,4 - Aryl Oxadiazole Derivatives
Musmade Deepak S1*, Shashikant PR2 and Yalgatti SM3
1Department of Pharmaceutical Chemistry, Research Scholar Jawaharlal Nehru Technological University, India
2Department of Pharmaceutical Chemistry, Adv. Abasaheb Kakade's College of Pharmacy, India
3Head, Department of Pharmaceutical chemistry, Srikrupa Institute of Pharmaceutical Sciences, India
*Corresponding author: Musmade Deepak S, Department of Pharmaceutical chemistry, SRES's Sanjivani College of Pharmaceutical Education and Research, Kopargaon, MS, India
Received: January 21, 2019; Accepted: February 19, 2019; Published: February 26, 2019
Abstract
The search for potent bioactive agents in pharmaceutical industry has been directed towards the nitrogen containing heterocycles. In recent days the researchers think about synthesis of bridged derivatives of nitrogen containing heterocycles. The present study reports the synthesis of a library new 1,3,4-Aryl oxadiazole derivatives. The structures of newly synthesized compounds were established using IR, 1H-NMR, Mass and elemental analysis. The newly synthesized compounds were screened for antimicrobial activity using cup-plate agar diffusion method some of the compounds shows promising antibacterial and antifungal activity. Also subjected for in-vitro anti-inflammatory activity using protein denaturation method. Some of the compounds show significant anti-inflammatory activity.
Keywords: 1,3,4-aryl-oxadiazole; Anti-microbial activity; Anti-inflammatory activity; Protein denaturation
Introduction
Mefenamic acid and Diclofenac are the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) which are mostly useful for the treatment of pain and threshold. It mostly acts through the inhibition of prostaglandin synthesis by inhibition of Cyclooxygenase (COX). It also exhibit bacteriostatic activity by inhibiting bacterial DNA synthesis. The long-term use of this agents may lead to the development of gastrointestinal ulceration, bleeding and in some cases renal disorders. Chronic use of non-steroidal anti-inflammatory drugs may elicit the appreciable gastro-intestinal toxicity. With the aim of improving safety, profile of NSAIDs chemical modification on these agents had been carried out. It has also been reported that compounds containing some substituted 1,3,4-aryl-oxadiazole moiety possess various biological activities likes antimicrobial activity [1], Anti-inflammatory activity [2], GOT, GPT AND c-GT inhibitory activity [3], Anti-cancer activity [4], Haemolytic activity [5], Antioxidant activity [6], Inhibitors of GSK-3b Kinase [7], Monoamine Oxidase (MAO) inhibitors [8], Anti-tubercular activity [9], Tubulin inhibitors [10], Lipoxygenase inhibitors [11], etc. By considering the above facts in this research, we had replaced the carboxylic acid moiety of Mefenamic acid and Diclofenac by substituted 1,3,4- aryl Oxadiazoles.
Materials and Methods
Material
All the chemicals required for the synthesis were purchased from Modern science, Nashik and are of AR grade.
Methods
In-vitro anti-inflammatory activity
Inhibition of protein denaturation
The standard drug and synthesized compounds were dissolved in minimum quantity of Dimethyl Formamide (DMF) and diluted with phosphate buffer (0.2 M, pH 7.4). Final concentration of DMF in all solution was less than 2.5%. Test solution (1mL) containing different concentrations of drug was mixed with 1 mL of 1mM albumin solution in phosphate buffer and incubated at 27° + 1°C in BOD incubator for 15 min. Denaturation was induced by keeping the reaction mixture at 60° + 1°C in water bath for 10 min. After cooling, the turbidity was measured at 660 nm (UV-Visible Spectrophotometer). Percentage of inhibition of denaturation was calculated from control where no drug was added. Each experiment was done in triplicate and average is taken. The Ibuprofen was use as standard drug. The percentage inhibition of denaturation was calculated by using following formula.
% of Inhibition = 100 × [1- Vt / Vc]
Where,
Vt = Mean absorbance of test sample.
Vc = Mean absorbance of control
Antibacterial Activity
Anti-bacterial study was carried out by using Cup-plate Agar diffusion method. The synthesized derivatives were tested in vitro for their anti-bacterial activity against E.coli (NCTC 10418), S. Aureus (NCTC 6571) and B. subtilis, which are pathogenic to human beings. Leavofloxacin had been used as a standard drug.
Anti-Fungal Activity
Anti-fungal activity was carried out by using Cup-Plate Agar diffusion method using nutrient agar as a culture media. The synthesized compounds were tested against Candida albicans (ATCC 10231) and Aspergilus niger (ATCC 16404). Amphotericin B had been used as a standard drug.
The synthesized compounds were dissolved in DMF and activities were carried out at a concentration of 200 µg/ml.
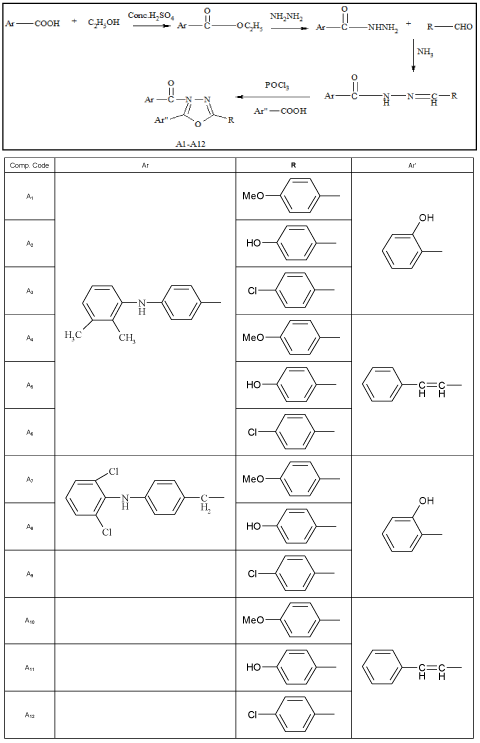
Scheme
Experimental
Melting points were determined in open capillary method and are uncorrected. Purity of the compound was checked on Silica gel TLC plates. IR spectra were recorded on Thermo Nicolate IR 200 spectrophotometer using KBr disc method. 1H NMR spectra were recorded on Bruker AMX-400, DMSO ?6 as solvent and TMS as internal standard. Combustion analyses were found to be within the limits of permissible errors.
Synthesis of Schiff’s bases from acid hydrazide and aromatic aldehyde: 0.01 mole of an acid hydrazide was dissolved in 10 ml of water along with little ammonia and stirred continuously with drop wise addition of 0.01 moles an aromatic aldehyde until a solid mass is obtained. Filter the precipitate and recrystallized from methanol [12].
Synthesis of 1, 3, 4-aryl Oxadiazoles from Schiff’s bases (A1-A12): 0.01 mole of an aromatic acid was dissolved in POCl3 in a fuming cupboard with continuous stirring until a uniform solution had been formed. After which 0.01 mole a schiffs base is added and temperature of a reaction mixture raised up to 150°C reflux continued for 2 hrs. Cooled and product is reprecipitated with addition of sodium bicarbonate and recrystallized using methanol to offer title compounds. Purity of synthesized compounds was checked using TLC. (Mobile phase: Toluene: methanol-3:1) [13,14].
Comp.code
Molecular formula
Mole. Wt.
M.P. (°C)
Elemental analysis Found (Cald.)
Rf Value
% Yield
C
H
N
A1
C30H27N3O4
493.6
271-273
73.01 -72.89
5.51-5.21
7.51 -7.23
0.48
54
A2
C29H25N3O4
479.5
236-241
72.64 -72.38
5.25-4.96
8.76 -8.39
0.61
51
A3
C29H24ClN3O3
498
238-242
69.95 -69.68
4.86-4.39
8.44 -8.25
0.64
62
A4
C32H29N3O3
503.6
268-273
76.32 -76.03
5.8-5.58
8.34 -8.13
0.49
64
A5
C31H27N3O3
489.6
301-305
76.05 -75.89
5.56-5.28
8.58 -8.31
0.58
68
A6
C32H28ClN3O2
532
318-323
73.62 -73.26
5.41-5.12
8.05 -7.86
0.61
64
A7
C29H23Cl2N3O4
548.4
308-313
63.51 -63.21
4.23-3.98
7.66 -7.38
0.6
78
A8
C28H21Cl2N3O4
534.4
287-293
62.93 -62.59
3.96-3.68
7.86 -7.64
0.54
58
A9
C28H20Cl3N3O3
552.9
309-315
60.83 -60.59
3.65-3.29
7.6 -7.38
0.57
53
A10
C31H25Cl2N3O3
558.5
278-283
66.67 -66.31
4.51-4.25
7.52 -7.21
0.48
57
A11
C30H23Cl2N3O3
544.4
272-277
66.18 -65.98
4.26 -3.98
7.72 -7.28
0.57
59
A12
C30H22Cl3N3O2
562.9
269-273
64.02-63.85
3.94 -3.58
7.47 -7.14
0.53
61
Table 1: Analytical data of synthesized compounds (A1-A12).
Spectral Data
Spectral Data: A1: IR (cm-1) KBr disc: 3250.15 –NH str.; 3256.23 –OH str.; 3002.58 Ar-CH str.; 2854.36 –CH3 str.; 1684.69 –CONH str.; 1556.24 –C=N str.; 1120.36 –C-O-C str.; 1H-NMR (ppm): 6.4-7.2 15H of phenyl; 6.0 1H of 1,3,4-oxadiazole; 5.4 1H of –OH; 5.2 1H of –NH; 1.2-2.6 9H of –CH3, m/e (100%): 493.
A2: IR (cm-1) KBr disc: 3260.25 –NH str.; 3185.36 –OH str.; 2986.37 Ar-CH str.; 2834.29 –CH3 str.; 1689.28 –CONH str.; 1564.32 –C=N str.; 1089.25 –C-O-C str.; 1H-NMR (ppm): 6.2-7.8 15H of phenyl; 6.2 1H of 1,3,4-oxadiazole; 5.6 2H of –OH; 5.0 1H of –NH; 1.2-2.6 6H of –CH3, m/e (100%): 479.
A3: IR (cm-1) KBr disc: 3245.25 –NH str3220.23 –OH str.; 3000.14 Ar-CH str.; 2810.37 –CH3 str.; 1685.23 –CONH str.; 1575.24 –C=N str.; 1059.51 –C-O-C str.; 1H-NMR (ppm): 6.1-7.6 15H of phenyl; 6.1 1H of 1,3,4-oxadiazole; 5.4 2H of –OH; 4.8 1H of –NH; 0.8-1.6 6H of –CH3, m/e (100%): 497.
A4: IR (cm-1) KBr disc: 3245.20 –NH str.; 3110.28 –CH=CH str.; 3025.14 Ar-CH str.; 2836.79 –CH3 str.; 1680.24 –CONH str.; 1556.29 –C=N str.; 1060.58 –C-O-C str.; 1H-NMR (ppm): 6.4-7.8 16H of phenyl; 6.2-6.4 2H of –CH=CH; 6.0 1H of 1,3,4-oxadiazole; 5.0 1H of –NH; 2.1-3.8 9H of –CH3, m/e (100%):503.
A5: IR (cm-1) KBr disc: 3240.27 –NH str.; 3220.28 –OH str.; 3125.86 –CH=CH str.; 2984.36 Ar-CH str.; 2815.34 –CH3 str.; 1687.24 –CONH str.; 1575.69 –C=N str.; 1035.28 –C-O-C str.; 1H-NMR (ppm): 6.2-7.6 16H of phenyl; 6.2-6.4 2H of –CH=CH; 6.0 1H of 1,3,4-oxadiazole; 5.4 1H of –OH; 5.0 1H of –NH; 2.1-2.6 6H of –CH3, m/e (100%): 489.
A6: IR (cm-1) KBr disc: 3250.48 –NH str.; 3184.26 –CH=CH str.; 3025.38 Ar-CH str.; 2826.38 –CH3 str.; 1690.27 –CONH str.; 1558.34 –C=N str.; 1039.34 –C-O-C str.; 987.25 –C-Cl bend 1H-NMR (ppm): 6.4-7.8 16H of phenyl; 6.2-6.4 2H of –CH=CH; 6.2 1H of 1,3,4-oxadiazole; 5.0 1H of –NH; 2.0-2.6 6H of –CH3, m/e (100%): 532.
A7: IR (cm-1) KBr disc: 3286.84 –NH str.; 3220.54 –OH str.; 3058.48 Ar-CH str.; 2856.37 –CH3 str.; 1694.25 –CONH str.; 1556.32 –C=N str.; 1025.39 –C-O-C str.;965.38 –C-Cl bend 1H-NMR (ppm): 6.2-7.8 15H of phenyl; 6.2 1H of 1,3,4-oxadiazole; 5.4 1H of –OH; 5.0 1H of –NH; 1.2-1.6 5H of –CH3, m/e (100%): 548.
A8: IR (cm-1) KBr disc: 3265.28 –NH str.; 3226.48 –OH str.; 3012.35 Ar-CH str.; 2825.36 –CH3 str.; 1686.26 –CONH str.; 1570.39 –C=N str.; 1070.38 –C-O-C str.;960.35 –C-Cl bend 1H-NMR (ppm): 6.4-7.8 15H of phenyl; 6.0 1H of 1,3,4-oxadiazole; 5.6 2H of –OH; 5.0 1H of –NH; 1.2-1.6 2H of –CH2, m/e (100%): 534.
A9: IR (cm-1) KBr disc: 3265.24 –NH str.; 3225.69 –OH str.; 2986.37 Ar-CH str.; 2830.35 –CH3 str.; 1684.38 –CONH str.; 1572.35 –C=N str.; 1085.34 –C-O-C str.;968.35 –C-Cl bend 1H-NMR (ppm): 6.4-7.6 15H of phenyl; 6.0 1H of 1,3,4-oxadiazole; 5.4 1H of –OH; 5.0 1H of –NH; 1.2-1.4 2H of –CH2, m/e (100%): 552.
A10: IR (cm-1) KBr disc: 3245.68 –NH str.; 3226.35 –CH=CH str.; 3025.69 Ar-CH str.; 2846.38 –CH3 str.; 1684.37 –CONH str.; 1576.34 –C=N str.; 1065.48 –C-O-C str.;967.28 –C-Cl bend 1H-NMR (ppm): 6.2-7.8 16H of phenyl; 6.2-6.6 2H of –CH=CH; 6.2 1H of 1,3,4-oxadiazole; 5.4 1H of –OH; 5.0 1H of –NH; 1.2-1.6 5H of –CH3, m/e (100%): 558.
A11: IR (cm-1) KBr disc: 3246.85 –NH str.; 3235.36 –CH=CH str.; 3226.34 –OH str.; 3025.61 Ar-CH str.; 2856.30 –CH3 str.; 1685.64 –CONH str.; 1565.32 –C=N str.; 1075.30 –C-O-C str.; 986.25 –C-Cl bend 1H-NMR (ppm): 6.4-7.8 16 H of phenyl; 6.4-6.6 2H of –CH=CH; 6.2 1H of 1,3,4-oxadiazole; 5.4 1H of –OH; 5.0 1H of –NH; 1.2-1.4 2H of –CH2, m/e (100%): 544.
A12: IR (cm-1) KBr disc: 3255.68 –NH str.; 3247.68 –CH=CH str.; 3025.64 Ar-CH str.; 2810.34 –CH3 str.; 1687.32 –CONH str.; 1576.24 –C=N str.; 1085.25 –C-O-C str.; 976.38 –C-Cl bend 1H-NMR (ppm): 6.2-7.6 16 H of phenyl; 6.4-6.6 2H of –CH=CH; 6.2 1H of 1,3,4-oxadiazole; 5.0 1H of –NH; 1.2-1.4 2H of –CH2, m/e (100%): 562.
Results and Discussions
The structures of the synthesized derivatives of 1, 3, 4-aryl-oxadiazoles (A1-A12) were established by IR, 1H-NMR, Mass spectra and elemental analysis. The purity of synthesized compounds had been checked on TLC plates using Toluene: Methanol (3:1) as a mobile phase. The IR, 1H-NMR and Mass data reported in manuscript under section of spectral data. The IR spectra shows absorption bands like 3250-3280cm-1 (-NH str.), 3220-3250 cm-1 (-OH str.), 2980-3050 cm-1 (Aromatic –CH str.), 2840-2880 cm-1 (aliphatic –CH str.), 1685-1695 cm-1 (-CONH str.), 1550-1585 cm-1 (-C=N str.), 1030-1080 (-C-O-C str.) which are characteristic feature of 1,3,4-aryl-oxadiazoles. 1H-NMR shows the peaks in 6.4-7.8 (Aromatic H), 6.0-6.2 (H of 1,3,4-oxadiazole), 5.4-5.6 (H of –OH group), 5.0 (H of –NH), 1.2-2.6 (H of –CH3 substituent of phenyl ring).
The synthesized compounds were subjected for in vitro anti-inflammatory activity. Out of twelve compounds, the compounds A2, A4 and A10 had shown significant anti-inflammatory activity. The structural features of the compounds like presence of electron donating group likes –CH3, -OCH3 along with hydroxyl (-OH) group was thought to increase the biological activity. While other compounds which possess electron withdrawing substituents like –Cl, -OH might be responsible for decrease in activity. Some derivatives which contains a –CH=CH linkage due to impartation of unsaturation character in the compounds also responsible for increase in biological activities.
The synthesized compounds were subjected for anti bacterial activity. Out of twelve compounds the compounds A3, A4,A5 and A10 had shown significant antibacterial activity. The structural features of the compounds like presence of electron donating group likes –CH3, -OCH3 along with hydroxyl group was thought to increase the biological activity. While other compounds which possess electron withdrawing substituents like –Cl, might be responsible for decrease in activity. In case of anti fungal compounds A1, A2,A6 and A11 shows significant activity as they posses higher percentage of electron donating groups like –CH3, -OCH3 which might increase the antifungal activity of these derivatives beside this these derivatives also contains a chloride linkage which increases the binding of drugs to the receptors this also responsible for increase in biological activities. Mostly Diclofenac derivatives shows significant antimicrobial activity as it brings inhibition of bacterial DNA synthesis.
Acknowledgement
Authors wish to express their sincere thanks to Hon. Shri. Amit Dada Kolhe saheb, Managing Trustee, Sanjivani Rural Education Society, Kopargaon for their constant support and encouragement.
References
- Andrea P, Piccionello PA, Musumeci R, Cocuzza C, Fortuna GC, Guarcello A, et al. Synthesis and preliminary antibacterial evaluation of Linezolid-like 1,2,4-oxadiazole derivatives. Eur J Med Chem. 2012; 50: 441-448.
- Desai NC, Amit DM, Rajpara KM, Yogesh RM. Synthesis and antimicrobial screening of 1,3,4-oxadiazole and clubbed thiophene derivatives. Journal of Saudi Chemical Society. 2014; 18: 255-261.
- Taher TA, Georgey HH, El-Subbagh IH. Novel 1,3,4-heterodiazole analogues: Synthesis and in-vitro antitumor activity. European Journal of Medicinal Chemistry. 2012; 47: 445-451.
- Chen JC, Bao-An S, Yang S, Xu FG, Bhadury SP, Jin LH, et al. Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole and 5- (3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorganic & Medicinal Chemistry. 2007; 15: 3981-3989.
- Samreen G, Aziz-ur-Rehman, Abbasi AM, Mohammed KK, Khadija N, Siddiqa A, et al. Synthesis, antimicrobial evaluation and haemolytic activity of 2-[[5-alkyl/aralkyl substituted-1,3,4- oxadiazol-2-yl]thio]-N-[4-(4-morpholinyl) phenyl] acetamide derivatives. Journal of Saudi Chemical Society. 2014; 015: 156-174.
- Padmavathi V, Dinneswara GR, Nagi SR, Mahesh K. Synthesis and biological activity of 2-(bis((1,3,4-oxadiazolyl/1,3,4-thiadiazolyl) methylthio) methylene) malononitriles. European Journal of Medicinal Chemistry. 2011; 46: 1367-1373.
- Monte LF, Kramer T, Gu J, Brodrecht M, Pilakowski J, Fuertes A, et al. Structure-based optimization of oxadiazole-based GSK-3 inhibitors. Eur J Med Chem. 2013; 61: 26-40.
- Ke S, Li Z, Qian X. 1,3,4-Oxadiazole-3(2H)-carboxamide derivatives as potential novel class of monoamine oxidase (MAO) inhibitors: Synthesis, evaluation, and role of urea moiety. Bioorganic & Medicinal Chemistry. 2008; 16: 7565-7572.
- Macaev F, Rusu G, Pogrebnoi S, Gudima A, Stingaci E, Vlad L, et al. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure–anti-mycobacterial activities. Bioorganic & Medicinal Chemistry. 2005; 13: 4842-4850.
- Andrei GA, Sosnov VA, Krasavin M, Nguyen LT, Hamel E. Identification of diaryl 5-amino-1,2,4-oxadiazoles as tubulin inhibitors: The special case of 3-(2-fluorophenyl)-5- (4-methoxyphenyl)amino-1,2,4-oxadiazole. Bioorganic & Medicinal Chemistry Letters. 2013; 23: 1262-1268.
- Aziz-ur-Rehman, Fatima A, Abbasi AM, Rasool S, Malik A, Ashraf M, et al. Synthesis of new N-(5-chloro-2-methoxyphenyl)-4-(5-substituted-1,3,4-oxadiazol-2-ylthio)butanamide derivatives as suitable lipoxygenase inhibitors. Journal of Saudi Chemical Society. 2013; 016: 156-174.
- Pattan SR, Deepak SM. Synthesis, antimicrobial and antitubercular activity of some novel [3-isonicotinoyl-5-(4-substituted)-2,3-dihydro-1,3,4-oxadiazol-2-yl and substituted 5-(pyridine-4-yl)-1,3,4-oxadiazole-2-thiole derivatives. Indian J Chemistry. 2013; 293-299.
- Chandrakantha B, Prakash S, Nambiyar V, Isloor N, Isloor AM. Synthesis, characterization and biological activity of some new 1,3,4-oxadiazole bearing 2-flouro-4-methoxy phenyl moiety. European Journal of Medicinal Chemistry. 2010; 45: 1206-1210.
- Pattan SR, Pattan JS, Musmade DS, Vetal SS, Pansare KD, Baheti DG, et al. Synthesis and evaluation of some substituted aryl oxadiazole and mercapto oxadiazole derivatives for antifungal, antimicrobial and anti tubercular activities, Indian Drugs. 2012; 49: 12-20.