
Research Article
Austin J Anal Pharm Chem. 2019; 6(1): 1112.
Developing a Highly Validated and Sensitive HPLC Method for Simultaneous Estimation of Oxytetracycline, Tinidazole and Esomeprazole in their Dosage Forms
Sebaiy MM1*, Hassan WS2, Saad MZ3, Zareh MM3 and Elhennawy ME3
¹Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
²Department of Analytical Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
³Department of Chemistry, Faculty of Science, Zagazig University, Zagazig, Egypt
*Corresponding author: Mahmoud M. Sebaiy, Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
Received: February 04, 2019; Accepted: April 16, 2019 Published: April 23, 2019
Abstract
An RP-HPLC method had been developed and validated for rapid simultaneous separation and determination of oxytetracycline, tinidazole and esomeprazole in their dosage forms within 6 minutes. Separation was carried out on a Kinetex Core-Shell (5μm, 150 x 4.60 mm) using an isocratic binary mobile phase of ACN: 0.025M KH2PO4 adjusted to pH 3.50 using orthophosphoric acid (25:75, v/v), filtered and degassed using 0.45μm membrane filter at ambient temperature. The flow rate was 1.00mL/min and maximum absorption was measured using DAD detector at 285nm. The retention times of oxytetracycline, tinidazole and esomeprazole were recorded to be 1.86, 2.61 and 6.07 minutes respectively, indicating a very short analysis time. Limits of detection were reported to be 0.07, 0.14 and 0.08 μg/ml for oxytetracycline, tinidazole and esomeprazole, respectively, showing a high degree of the method sensitivity. Validation parameters were then applied on the method according to ICH guidelines for the determination of the drugs in their dosage forms and showed highly precise recoveries.
Keywords: RP-HPLC; Oxytetracycline; Tinidazole; Esomeprazole; Dosage forms
Introduction
Oxytetracycline (OXY), chemically, is 4-(dimethylamino)- 1,5,6,10,11,12a-hexahydroxy-6-methyl-3,12-dioxo-4,4a,5,5atetrahydrotetracene- 2-carboxamide (Figure 1). Oxytetracycline is indicated for treatment of infections caused by a variety of gram positive and gram negative microorganisms including Mycoplasma pneumoniae, Pasteurella pestis, Escherichia coli, Haemophilus influenzae (respiratory infections), and Diplococcus pneumoniae [1]. Tinidazole (TIN), chemically, 1-(2-ethylsulfonylethyl)-2- methyl-5-nitroimidazole (Figure 1). It is anti-infective agent and it is a synthetic antiprotozoal agent. Tinidazole demonstrates activity both in vitro and in clinical infections against many protozoa like Trichomonas vaginalis, Giardia duodenalis and Entamoeba histolytica. On the other hand, it does not appear to have activity against most strains of vaginal lactobacilli [2]. Esomeprazole (ESM), chemically, is 6-methoxy-2-[(S)-(4-methoxy-3,5-dimethylpyridin- 2-yl)methylsulfinyl]-1H-benzimidazole (Figure 1). Esomeprazole is the S-isomer of omeprazole, with gastric proton pump inhibitor activity. In the acidic compartment of parietal cells, esomeprazole is protonated and converted into the active achiral sulfenamide which forms one or more covalent disulfide bonds with the proton pump hydrogen-potassium adenosine triphosphatase (H+/K+ ATPase), thereby inhibiting its activity and the parietal cell secretion of H+ ions into the gastric lumen, the final step in gastric acid production [3]. The mixture of the three drugs is now commercially used on a large scale for treatment of the duodenal ulcers caused by the anaerobic bacteria Helicobacter pylori.
Figure 1: Chemical structures of oxytetracycline (OXY), tinidazole (TIN) and
esomeprazole (ESM).
Various analytical techniques have been employed for the estimation of oxytetracycline, tinidazole and esomeprazole such as UV-vis spectrophotometry [4-5], high-performance liquid chromatography [6-14], charge transfer methods [15] and potentiometric methods [16-17].
To the best of our knowledge and comprehensive survey, oxytetracycline, tinidazole and esomeprazole mixture was not determined before by chromatographic techniques neither in pharmaceutical nor in biological samples despite their synergistic action. As such, the present work introduces a simple, rapid, reproducible and sensitive chromatographic method for the determination of oxytetracycline, tinidazole and esomeprazole in their pure forms and in their tablet dosage forms.
Experimental
Apparatus
Agilent 1200® HPLC instrument (Germany) with a Kinetex (5μm, 150 x 4.60 mm), DAD absorbance detector, HPLC QUAT pumps and connected to PC computer loaded with Agilent 1200 software.
Labomed® Spectro UV-VIS Double Beam (UVD-2950) Spectrophotometer with matched 1cm quartz cells and connected to windows compatible computer using UV Win 5 Software v6.
Jenway 4330 pH-meter (UK) for pH adjustment.
Materials and reagents
All solvents and reagents were of an HPLC analytical grade ( acetonitrile, potassium dihydrogen phosphate and ortho-phosphoric acid were supported from Fisher Scientific, England).
Oxytetracycline, Tinidazole and Esomeprazole were kindly provided by different Egyptian companies like Egyptian Company for Pharmaceutical & Chemical Industries (EIPICO), Egyphar Company and Delta pharm Company. Standard solutions of 200μg/mL were prepared by dissolving 20mg of each pure drug in 100ml of the mobile phase.
Mobile phase was a freshly prepared Isocratic binary mobile phase of ACN : 0.025M KH2PO4 adjusted to pH 3.50 using orthophosphoric acid (25 : 75, v/v), filtered and degassed using 0.45μm membrane filter.
Unimycin® tablets (Unipharma, Egypt), Fasigyn® tablets (Pfizer, Egypt) and Ezogast® tablets (Copad, Egypt) were labeled to contain 500mg oxytetracycline, 500 mg tinidazole and 40 mg esomeprazole, respectively.
Procedures
Preparation of standard calibration curves: Appropriate mixed dilutions of Oxytetracycline, Tinidazole and Esomeprazole standard stock solutions were prepared in 10ml volumetric flasks to get final concentrations of 10, 25, 40, 50, 75 and 100 μg/mL for all drugs. A 20μl of each mixture was injected into the column and the chromatogram was obtained at 285nm. A graph was plotted as concentration of drugs against response (peak area). Regarding validation QC samples, concentrations of 20, 50 and 90 μg/mL were selected as low (LQC), medium (MQC) and high (HQC) levels, respectively.
Pharmaceutical dosages preparation procedure: Other than 5 tablets of Unimycin®, Fasigyn® and Ezogast® tablets were weighed and powdered. An accurately volume or amounts equivalent to 20mg of each drug were dissolved in the mobile phase, filtered into 100ml measuring flasks and completed to volume with the mobile phase. The procedure was then completed as mentioned above under the general procedure 2.3.1.
Results and Discussion
Optimization of chromatographic conditions
All chromatographic conditions are illustrated in Table 1. Spectroscopic analysis of the drugs in the range of 200-400 nm showed that oxytetracycline, tinidazole and esomeprazole have UV absorbance maxima (λmax) at 264, 310 and 305 nm, respectively as depicted in Figure 2. Therefore, the chromatographic detection was performed at 285nm as a compromise and an appropriate wavelength for the three drugs using the DAD detector. The method was performed on a Kinetex Core-Shell (5μm, 150 x 4.60 mm).
Parameters
Conditions
Column
Kinetex Core-Shell (5 µm, 150 x 4.60 mm)
Mobile phase
Isocratic binary mobile phase of ACN: 0.025M KH2PO4 adjusted to pH 3.50 using ortho- phosphoric acid (25:75, v/v), filtered and degassed using 0.45µm membrane filter.
UV detection, nm
285
Flow rate, ml/min
1
Injected volume, µl
20
Temperature
Ambient
Table 1: Chromatographic Conditions for the proposed method.
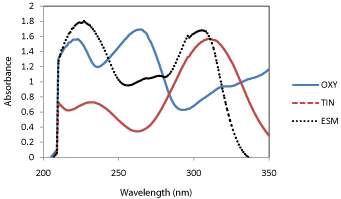
Figure 2: Overlain spectra of oxytetracycline (OXY___), tinidazole (TIN--
--) and esmoprazole (ESM.......) at equal concentrations of 2.5μg/mL, and
maximum wavelengths of 264, 310 and 305 nm, respectively.
Furthermore, under several trials of mobile phase optimization
regarding its composition ratio and pH, it was observed that the
optimized mobile phase was determined as a mixture of ACN: 0.025M
KH2PO4 adjusted to pH 3.50 using ortho-phosphoric acid (25:75, v/v)
at a flow rate of 1ml/min. Under these conditions, oxytetracycline,
tinidazole and esomeprazole in pure form can be separated and
eluted at 1.86, 2.61 and 6.07 minutes as illustrated in Figure 3A and in
dosage form at 1.86, 2.62 and 6.11 minutes, respectively as illustrated
in Figure 3B. However, in both cases, the optimum mobile phase showed symmetrical peaks (0.81 < T < 1.08), capacity factor (1
Parameters
Pure sample
Tablets dosage form
Reference values [19]
OXY
TIN
ESM
OXY
TIN
ESM
Retention time, tr
1.86
2.61
6.07
1.86
2.62
6.11
Capacity factor, k'
1.06
1.9
5.74
1.06
1.91
5.78
Accepted k' value (1-10)
Peak asymmetry (Tailing factor, T)
0.84
0.81
1.07
0.85
0.83
1.08
Accepted T value = 2
Therotical plates, N
6839
5781
14093
6839
5708
14101
Accepted N value > 2000
Resolution, Rs
---
6.6
20.19
---
6.6
20.24
Accepted value > 2
Selectivity (Separation factor, a)
---
1.4
2.32
---
1.41
2.33
Table 2: System suitability parameters for oxytetracycline (OXY), tinidazole (TIN) and esomeprazole (ESM) in both pure and pharmaceutical samples.
Method validation
The method validation was performed according to international conference of harmonization guidelines (ICH) [18].
Linearity: Six different concentrations of the drug mixture were specified for linearity studies. The calibration curves obtained by plotting peak area against concentration showed linearity in the concentration range of 10 - 100 μg/mL for all drugs (Table 3). Linear regression equations of oxytetracycline, tinidazole and esomeprazole were found to be y = 30.579x + 26.055, y = 18.558x + 20.07, and y = 29.99x + 37.232, respectively and the regression coefficient values (r) were calculated to be 0.999 for oxytetracycline and tinidazole and 1 for esomeprazole indicating a high degree of linearity (Figure 4).
OXY
TIN
ESM
Conc. taken (µg/mL )
Conc. found (µg/mL )
Recovery
% Accuracy
Conc. taken (µg/mL )
Conc. found (µg/mL )
Recovery
% Accuracy
Conc. taken (µg/mL )
Conc. found (µg/mL )
Recovery
% Accuracy
10
9.87
98.7
-1.25
10
9.98
99.86
-0.13
10
9.86
98.6
-1.37
25
24.8
99.27
-0.72
25
25.02
100.1
0.1
25
24.9
99.65
-0.34
40
40.35
100.88
0.88
40
40.56
101.4
1.4
40
40.45
101.13
1.13
50
50.26
100.5
0.52
50
49.3
98.6
-1.39
50
49.86
99.7
-0.26
75
74.6
99.5
-0.5
75
75.01
100.02
0.02
75
74.9
99.9
-0.09
100
100.06
100.06
0.06
100
100.1
100.1
0.1
100
99.97
99.97
-0.02
Mean
99.83
-0.16
100.01
0.019
99.83
-0.16
SD
0.8
0.89
0.8
CV (%)
0.8
0.89
0.8
SE
0.32
0.36
0.32
Variance
0.64
0.79
0.64
Slope
30.57
18.55
29.99
LOD (µg/mL)
0.07
0.14
0.08
LOQ (µg/mL)
0.26
0.48
0.27
Table 3: Analytical merits for determination of oxytetracycline (OXY), tinidazole (TIN) and esomeprazole (ESM) in pure samples using the proposed method.
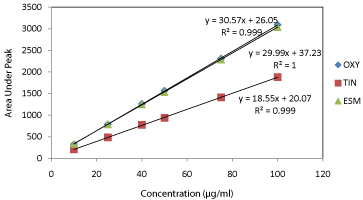
Figure 4: Calibration curves for authentic mixture of oxytetracycline (OXY),
tinidazole (TIN) and esomeprazole (ESM) using the proposed HPLC method.
Accuracy: The accuracy of the method was determined by investigating the recoveries of commercial formulations at concentration of 50μg/mL (three replicates). From the amount of the drug estimated, the percentage recovery was calculated and the results shown in Table 4 are indicating excellent recoveries for all drugs.
Conc. (µg/mL )
Found Conc. (µg/mL )
Mean ± SD
CV
accuracy
OXY (Unimycin®)
50
48.8
97.65 ± 0.75
0.59
-2.3
(n=3)
TIN (Fasigyn®)
50
50.01
100.02 ± 0.06
0.06
0.03
(n=3)
ESM (Esogast ® )
50
49.69
99.38 ± 0.42
0.42
-0.6
(n=3)
Table 4: Determination of Unimycin ®, Fasigyn ® and Esogast ® dosage forms using the proposed method.
Precision: The precision of the method was evaluated according to intra-day and inter-day precision using validation QC samples at concentrations of 20, 50 and 90 μg/mL. Intra-day precision was evaluated in respect of both standard deviation (SD) and coefficient of variation (CV%) regarding three replicate determinations using the same solution containing pure drugs. The SD and CV% values (varied from 0.03 to 0.55) in Table 5 revealed the high precision of the method. For inter-day reproducibility, the day-to-day SD and CV% values were also in the acceptable range of 0.13 to 2.18 (Table 5). These results indeed show that the proposed method has an adequate precision in simultaneous determination of the three drugs in their pharmaceutical formulations.
Drugs
Concentrations (µg/mL )
Mean recovery ± SD
CV (%)
Intra-day runs (n=3)
OXY
20
98.27 ± 0.18
0.19
50
97.90 ± 0.54
0.55
90
99.59 ± 0.37
0.37
TIN
20
99.19 ± 0.04
0.05
50
99.30 ± 0.24
0.24
90
100.41 ± 0.40
0.4
ESM
20
98.04 ± 0.03
0.03
50
98.95 ± 0.22
0.22
90
100.15 ± 0.07
0.07
Inter-day runs (n=3)
OXY
20
96.02 ± 2.08
2.17
50
96.10 ± 2.10
2.18
90
98.36 ± 1.46
1.48
TIN
20
99.16 ± 0.13
0.13
50
99.60 ± 0.49
0.49
90
100.73 ± 0.70
0.7
ESM
20
98.28 ± 0.30
0.3
50
99.40 ± 0.29
0.29
90
100.50 ± 0.38
0.38
Table 5: Intra- and inter-day precision results of oxytetracycline (OXY), tinidazole (TIN) and esomeprazole (ESM) in pure samples using the proposed method.
Selectivity and specificity: Selectivity of the method was checked by injecting the solutions of oxytetracycline, tinidazole and esomeprazole into the column separately where three sharp peaks were obtained at retention times of 1.86, 2.61 and 6.07 minutes, respectively, and these peaks were not obtained for the blank solution. Also, the specificity studies revealed that the presence of the excipients in the tablet formulations didn’t show any kind of impurity interference with the sharp and well-resolved peaks of the three drugs (Figure 3).
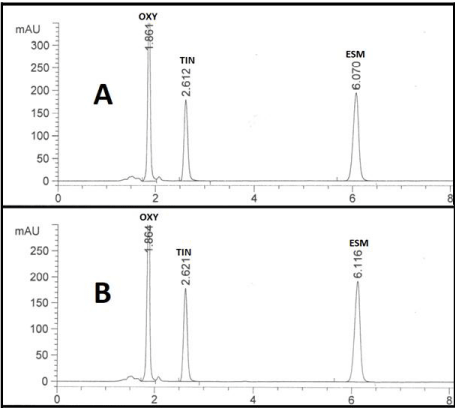
Figure 3: HPLC Chromatogram of (A) 50μg/mL authentic mixture of
oxytetracycline (OXY), tinidazole (TIN) and esomeprazole (ESM) and (B)
Unimycin®, Fasigyn® and Esogast® tablets mixture using Kinetex Core-Shell
(5μm, 150 x 4.60 mm) and a mobile phase of ACN: 0.025M KH2PO4 adjusted
to pH 3.50 using ortho-phosphoric acid (25:75, v/v). Other chromatographic
conditions are stated in Results and Discussion section 3.1. and Table 1.
Limits of detection and limits of quantification: For determining the limits of detection and quantitation, the method based on signalto- noise ratio (3:1 for LOD & 10:1 for LOQ) was adopted. Limits of detection were reported to be 0.07, 0.14 and 0.08 μg/mL, while limits of quantification were calculated to be 0.26, 0.48 and 0.27 μg/mL for oxytetracycline, tinidazole and esomeprazole, respectively (Table 3). These results show that the proposed method is highly sensitive and applicable not only for pharmaceutical analysis but also for pharmacokinetic and bioequivalence studies where detection of small concentrations is required.
Robustness: The robustness of the methods was evaluated by making deliberate subtle changes in the flow rate, mobile phase composition ratio and column temperature keeping the other chromatographic conditions constant. The changes effect was studied on the basis of percent recovery and standard deviation of all drugs. Table 6 shows that the changes had negligible influences on the results as revealed by small SD values for all applied changes.
Parameter
OXY
TIN
ESM
Mean recovery ± SD
CV (%)
Mean recovery ± SD
CV (%)
Mean recovery ± SD
CV (%)
Flow rate 0.95 ml
99.96 ± 0.98
0.98
101.06 ± 1.49
1.92
100.58 ± 1.90
1.94
Flow rate 1.05 ml
98.30 ± 3.42
3.48
99.37 ± 2.34
2.36
99.00 ± 2.23
2.25
ACN : Buffer
24 : 76
99.49 ± 0.87
0.87
100.10 ± 0.68
0.65
99.70 ± 0.85
0.86
ACN : Buffer
26 : 74
98.70 ± 2.50
2.55
100.18 ± 0.63
0.63
99.87 ± 0.80
0.8
Temp 28°c
99.17 ± 1.46
1.47
100.19 ± 0.62
0.61
99.84 ± 0.80
0.8
Temp 32°c
99.20 ± 1.40
1.4
100.10 ± 0.69
0.69
99.49 ± 1.20
1.2
Table 6: Results of the robustness for the determination of 50 μg/mL oxytetracycline (OXY), tinidazole (TIN) and esomeprazole (ESM) using the proposed method.
Analysis of pharmaceutical formulations: Unimycin®, Fasigyn® and Ezogast® pharmaceutical formulations containing oxytetracycline, tinidazole and esomeprazole, respectively, had been successfully analyzed by the proposed method. Excipients and impurities did not show interference indicating a high degree of specificity for the method. Results obtained were compared to those obtained by applying reference methods [7,10,12] where Student’s t-test and F-test were performed for comparison. Results shown in Table 7 indicated that calculated t and F values were less than tabulated ones for the three drugs which in turn indicate that there is no significant difference between proposed method and reference ones relative to precision and accuracy.
OXY (Unimycin®)
TIN (Fasigyn®)
ESM (Ezogast®)
Proposed method
Reference method [7]
Proposed method
Reference method [10]
Proposed method
Reference method [12]
N
3
3
3
3
3
5
Mean Recovery
100.02
100
99.57
99.04
99.87
99.86
SE
0.29
0.78
0.48
0.28
0.07
0.11
Variance
0.26
1.82
0.71
0.24
0.01
0.06
Student-t
0.02 (2.13)a
0.95 (2.13)a
0.07 (1.94)a
F-test
6.92 (19.00)b
2.95 (19.00)b
4.65 (19.25)b
a and b are the Theoretical Student t-values and F-ratios at p=0.05.
Table 7: Statistical analysis of results obtained by the proposed method applied on Unimycin® tablets, Fasigyn® tablets and Ezogast® tablets compared with reference methods.
Conclusion
The presented method was developed and validated for rapid and simultaneous estimation of oxytetracycline, tinidazole and esomeprazole within 6 minutes. The results obtained indicate that the proposed method is rapid, accurate, selective, robust and reproducible. Linearity was observed over a concentration range of 10 - 100 μg/mL for the three drugs. The method has been successfully applied for the analysis of marketed formulations Unimycin®, Fasigyn® and Ezogast® in respect of quality control, where low cost and fast analysis are essential.
Ethical Approval
This manuscript does not include any studies on human or animals.
References
- Ian C and Marilyn R. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol Mol Biol Rev. 2001; 65: 232–260.
- Sarah LC, Kiera LD, Shannon FH, Dino PP, Gary EG. Treatment of Infections Caused by Metronidazole-Resistant Trichomonas vaginalis. Clin Microbiol Rev. 2004; 17: 783–793.
- Vic V, Ravi V, Gregory O. Esomeprazole: A proton pump inhibitor. Expert review of gastroenterology & hepatology. 2009; 3: 15-27.
- Emara KM, Askal HF, Saleh GA. Spectrophotometric determination of tetracycline and oxytetracycline in pharmaceutical preparations. Talanta. 1991; 38: 1219-1222.
- Noura HA, Dina TE, Dalia RE, Mohamed AA and Saadia ME. Simultaneous Determination of Norfloxacin and Tinidazole Binary Mixture by Difference Spectroscopy. Int J Biomed Sci. 2011; 7: 137–144.
- Vitthal SH, Parag TB, Sachin SR, Vijay RP. Uv spectrophotometric estimation of esomeprazole and domperidone in tablet dosage form. Ind Res J Pharm Sci. 2016; 3: 804-810.
- Papadoyannis IN, Samanidou VF, Kovatsi LA. A rapid high performance liquid chromatographic (HPLC) assay for the determination of oxytetracycline in commercial pharmaceuticals. J Pharm Biomed Anal. 2000; 23: 275-280.
- Khan Nh, Roets E, Hoogmartens J, Vanderhaeghe H. Quantitative analysis of oxytetracycline and related substances by high-performance liquid chromatography. J Chromatogr A. 1987; 405; 229-245.
- Rajnarayana K, Chaluvadi MR, Alapati VR, Mada SR, Jayasagar G, Krishna DR. Validated HPLC method for the determination of tinidazole in human serum and its application in a clinical pharmacokinetic study. Pharmazie. 2002; 57: 535-537.
- Meshram DB, Bagade SB, Tajne MR. Simple HPLC method for simultaneous estimation of fluconazole and tinidazole in combined dose tablet. J Chromatogr Sci. 2009; 47: 885-888.
- Pasha K, Ali A, Bana S, Humair S. Reverse phase - HPLC method for the analysis of Tinidazole in pharmaceutical dosage form & bulk drug. Int J Pharmacy Pharm Sci. 2010; 2: 46-47.
- Ragab MA, El-Kimary E. High performance liquid chromatography with photo diode array for separation and analysis of naproxen and esomeprazole in presence of their chiral impurities: Enantiomeric purity determination in tablets. J Chromatogr A. 2017; 1497: 110-117.
- Jagan TSS, Varma DP, Bhavyasri K, Prasad K, Khagga M Jogia. H. HPLC method for determining esomeprazole and its related substances in pharmaceutical formulations. Asian J Chem. 2017; 29; 1360-1364.
- Yahdiana H, Ahmad EB. Method Validation of Esomeprazole Analysis in Human Plasma using High Performance Liquid Chromatography–Photodiode Array. J Young Pharm. 2017; 9: s24-s28.
- Khairi MSF. Analysis of Certain Tetracyclines and Oxytetracyclines through Charge Transfer Complexation. Amer J Pharmacol Toxicol. 2008; 3: 212-218.
- Guy B, Michel B, Luc L. Metal ion-tetracycline interactions in biological fluids. 2. potentiometric study of magnesium complexes with tetracycline, oxytetracycline, doxycycline, and minocycline, and discussion of their possible influence on the bioavailability of these antibiotics in blood plasma. J Inorg Biochem. 1983; 19: 1-18.
- Basavaiah K, Nagegowda P, Chandrashekar U. Determination of tinidazole by potentiometry, spectrophotometry and high performance liquid chromatography. CSIR. 2005; 12: 273-280.
- Guidance for Industry: Q2B Validation of Analytical Procedures: Methodology. International Conference of Harmonization (ICH). Nov.1996.
- CDER Center for Drug Evaluation and Research, Reviewer Guidance Validation of Chromatographic Methods. 1994.