
Research Article
Austin J Anal Pharm Chem. 2019; 6(1): 1113.
Simultaneous Determination of Some Selected Hypertension Drugs in Hospital Effluents by Using LCMS
Elhag DE*, Abdallah BS and Suliman SA
¹Institute of Research, UMST University, Khartoum, Sudan
*Corresponding author: Dhia Eldin Elhag, Institute of Research, UMST University, Khartoum, Sudan
Received: February 14, 2019; Accepted: April 19, 2019; Published: April 26, 2019
Abstract
A robust method was developed and validated for the determination of Bisoprolol, Losartan, Amlodipine, Atorvastatin and Candesartan antihypertension drugs in hospital wastewater. The method employs acetonitrile/formic acid as a mobile phase in a gradient mode, flow rate 1ml/min. The column used was ODS Shimpack 4.6*150*5μm and the injected volume was 5μL. Solid phase extraction was used for sample cleaning before analysis. Analysis time was less than seven minutes. The limits of quantitation (LOQ) ranges were 0.001-0.003 and 0.003-0.014 ug/ml, respectively. Acceptable recoveries were achieved for the drug standards. This new method proved to be simple, robust, precise and accurate.
Keywords: LCMS; Method development; Hypertension drugs
Introduction
Pharmaceuticals are emerging as potential pollutants of the environment and have become a considerable source of environmental contamination [1–4]. A number of therapeutic pharmaceuticals are used in large quantities and may be present in effluents at varying concentrations. The health impact of long-term exposure to mixtures of these compounds is unknown. Sources of pharmaceutical pollution of the environment include humans, hospitals, livestock and pharmaceutical industry. They can enter the waterways through their disposal and as a result can become a serious and persisting health hazard to human health, as well as to the life of the flora and fauna. The presence of pharmaceuticals in the environment raised great concern in the recent years regarding their potential impact as they can affect water quality by the increasing discharge of drugs. This issue has prompted numerous monitoring and assessment programs and studies [5–9].
The determination of pharmaceuticals at trace levels in various environmental matrices, especially water, is mainly attributable to the advancement of the sensitivity and accuracy of the new generations of analytical instrumentation. For instance, high performance liquid chromatography (HPLC) coupled to mass spectrometry, is a common technique of choice for various drug residues and pharmaceuticals [2,10–15]. Anti-Hypertensive drugs are pharmaceutical used to help control blood pressure. Untreated high blood pressure can lead to diseases of the heart, arteries, kidney damage, or stroke [16–18]. In this study, we report the development and validation of an HPLCMS method enabling to quantify simultaneously some selected antihypertensive drugs i.e. Bisoprolol, Losartan, Amlodipine, Atorvastatin and Candesartan.
Materials and Methods
Drugs standard Bisoprolol, Losartan, Amlodipine, Atorvastatin and Candesartan were kindly donated by Kingstone Pharmaceuticals, Sudan, Acetonitrile, (Carlo Erba, France), formic acid, acetone 99% triethylamine methanol (99%) sulphoric acid (98%), ethanol (99%) (SDFCL, India). Deionized water, filter paper (Watman 100 and 0.45 micron filter paper. All chemical were analytical or HPLC grade.
Preparation of standard stock solution
Exactly 0.01g from each standard was weighed separately in a beaker (50mL) and 10ml of the diluent was added. The standard was transferred to ultrasonic bath for 5min and then was completed to the mark to obtain a concentration of 200mg/ml. From this standard solution, 0.5ml were pipetted into 10ml volumetric flask and then completed to the mark by the diluent to obtain a final solution of a concentration of 10ug/ml. Further dilutions were made from this solutions for the different validation tests.
Samples pretreatment and clean up
Initially, 500 ml of each sample of wastewater was filtered through Watman filter paper (100) and then through 0.45 micron, then acidified to pH 3.0 by adding sulphuric acid. The sample was passed through C-18 cartridge activated with 5ml of methanol, 5ml methanol/water 50/50 (v/v) and 5ml of water at (pH 3). The cartridge was washed further with 5ml of acidified water (pH 3).The cartridge was eluted with 5ml of triethylamine (5% v/v) in methanol. The eluted solution was evaporated at normal room temperature (200C). Finally, sample volume was completed to 1ml by adding water/acetonitrile 95/5 (v/v).
Mobile phase preparation of mobile phase
Gradient elution mode was used in the analysis. Mobile phase A (1% formic acid) and mobile phase B was acetonitrile. The gradient program is shown in Table 1.
Time (min)
A
B
0.01
70
30
3
40
60
5
20
80
8
70
30
9
70
30
10
stop
Table 1: The Gradient elution program.
Preparation of the diluents: The diluent was made from formic acid 1% and acetonitrile (50%, 50%).
HPLC conditions: HPLC column was: ODS Shimpack 4.6*150*5μm,-flow rate : 1mL/min, injection volume: 5μL
MS condition: Drying gas: N2 15L/min, nebulizing gas N2 1.5L/ min, interface temperature: 3500C heat block: 2000C, the scan mode: +ve SIM, and the drugs selected ions (M+H) used in the analysis were as follows: Bisoprolol (326), Losartan (423.15), Amlodipine (409), Atorvastatin (559) and Candesartan (611).
Results and Discussion
LC-MS optimization
Several gradient programs were tried to achieve optimum separation of the entire drug standards. Gradient elution was necessary to avoid excessive retention. Well-resolved peaks were obtained within short analysis time. The positive and the negative electrospray ionization (ESI) modes were investigated for attaining the highest sensitivity during the method development. The full scan of the drugs mixture in positive mode showed that the signal-to- noise ratios obtained in this mode were higher than those of the in negative mode. Hence, positive mode was used to obtain the precursor ion [M+H] for the analysis. During the method development, the quadrupole mass analyzers operated in selected ion monitoring (SIM) mode where it monitors only a few mass-to- charge ratios. By setting the optimal chromatographic parameters and using electrospray ionization, the produced chromatogram and SIM spectra of the drug standards are shown in Figure 1. The retention times of Bisoprolol, Losartan, Amlodipine, Atorvastatin and Candesartan were 3.59, 4.71, 5.17, 6.00 and 6.49 min, respectively.
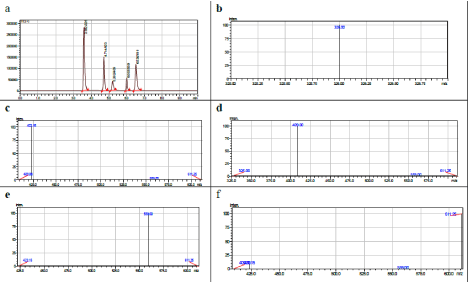
Figure 1: a) The chromatogram of the drugs; b) SIM spectra of Bisoprolol; c) Losartan; d) Amlodipine; e) Atorvastatin; f) Candesartan.
Method validation
Test method validation is a set of Comprehensive experiments that evaluate and document the performance of an assay. These experiments are designed to demonstrate the scientific validity of results produced by the method during routine sample analysis. Establishing that a test method consistently produces reliable analytical results is a critical element of assuring the quality and safety of pharmaceutical products. In this work validation was performed according to the ICH guidelines [19].
System suitability testing: As an essential part of the method development, system suitability was studied to interpret the chromatographic performance of the instrument. Resolution, tailing factor, number of theoretical plates, peak width and height equivalent to a theoretical plate (Table 2).
Theoretical plates
Tailing factor
Retention time
Resolution
Bisoprolol
10826
1.57
3.59
--
Losartan
22718
1.244
4.71
8.504
Amlodipine
12353
1.485
5.17
3.114
Atorvastatin
37869
1.479
6
5.731
Candesartan
17739
1.617
6.49
3.245
Table 2: System Suitability test results.
Specificity: Specificity is the ability to assess unequivocally the analyte in the presence of components which might be expected to be present. The chromatogram and peaks of interest were investigated for the presence of any interferent peaks. No such observations were noticed in the obtained chromatogram.
Linearity: Linearity is the ability of the method to return values that are directly proportional to the concentration of the drugs in the sample. The linearity plot was examined over a number of concentration levels (0.1, 0.4, 1, 1.5 and 2ug/ml) of the standard drug solutions. Each solution was injected in triplicate and average values were used as representative. Slope, intercept and correlation coefficients, the slope and the intercept, were calculated using Lab solution software. The data obtained and the regression lines obtained for each drug are shown in Table 3.
Drug
Regression equation
R2
Bisoprolol
y = 7E+06x + 1833.
R² = 0.998
Losartan
y = 3E+06x - 30225
R² = 0.998
Amlodipine
y = 1E+06x - 26297
R² = 0.998
Atorvastatin
y = 1E+06x - 22014
R² = 0.998
Candesartan
y = 4E+06x + 63628
R² = 0.998
Table 3: linearity regression equations of the drugs.
Accuracy and recovery: Recovery studies were performed to determine accuracy. A standard solution of Bisoprolol was were spiked with known amounts at 3 different levels (80%, 100% and 120%). The solutions were analyzed and the mean recovery was calculated. The process was repeated for the other drugs and the results obtained are shown in Table 4.
Drug
AVG RE 80%
AVG RE 100%
AVG RE 120 %
Bisoprolol
98.17
97.87
98.73
Losartan
98.8
98.31
98.85
Amlodipine
97.58
97.67
98.87
Atorvastatin
99.06
100.28
99.5
Candesartan
99.18
99.62
99.01
Table 4: Accuracy test results.
Intraday and interday precision: The precision of an analytical procedure expresses the closeness of agreement between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. Precision experiments included intraday and interday studies. Results were statistically evaluated including mean, standard error of mean, standard deviation, RSD%. The results obtained are shown in Table 5 and 6.
Drug
Bisoprolol
RSD
Amlodipine
Atorvastatin
Candesartan
Conc (ug/ml)
RSD
Losartan
RSD
RSD
RSD
Day1
0.4
1.1
0.5
0.76
1.03
0.67
1
1.6
1.25
0.44
0.73
1.5
1
2
1.1
0.76
1.25
0.49
0.83
9
Day2
0.4
0.7
0.77
1.37
1.3
1.22
1
1
1.3
1.4
0.66
1.14
0.58
8
2
1.1
1.05
1.79
1.35
0.38
9
Day3
0.4
1.5
1.15
1.37
0.52
0.94
9
1
1.6
1.24
1.32
0.97
1.06
5
2
0.8
0.66
1.3
0.92
0.68
2
Table 5: Intraday Precision test results.
Drug Conc (ug/ml)
Bisoprolol
RSD
Amlodipine
Atorvastatin
Candesartan
RSD
Losartan
RSD
RSD
RSD
0.4
0.9
0.89
1.26
0.83
1.09
3
1
0.8
1.4
1.13
1.08
0.87
2
1
2
0.6
1
0.82
1.45
0.92
3
7
Table 6: Interday precision test results.
Limits of detection and quantification: The calculated values were regarded as the lowest concentration for detection and quantitation (Table 7).
Drug
Selected
LOD
LOQ
Ion[M+H]
Bisoprolol
0.001
0.0103
326
0
Losartan
0.001
0.0142
423.15
4
Amlodipine
0.001
0.0099
409
0
Atorvastatin
0
0.0031
559
3
Candesartan
0.003
0.0319
611
2
Table 7: Limits of Detection and Quantification test results.
Robustness: Robustness was investigated by minor variation of the experimental conditions i.e. mobile phase composition, pH, and flow rate and column temperature intentionally. The experimental results did not show any significant difference from the initial parameters.
Solution stability: The solution stability test was performed by measuring the same samples after six hours. The results obtained in shown in Table 8.
Drug
Theortical plates
Tailing factor
Retention time
Resolution
RSD
Bisoprolol
0.19
9675
1.437
3.588
--
41
Losartan
4.71
0.46
21342
1.275
8.504
59
Amlodipine
0.41
11070
1.523
5.17
3.114
28
Atorvastatin
0.02
33399
1.385
5.999
5.731
75
Candesartan
0.31
15897
1.47
6.49
3.245
77
Table 8: Solution stability test results.
Analysis of samples: Using this method, water samples from a effluents and drinking water sample from two hospitals in Khartoum, Sudan were collected and treated as mentioned previously and finally analysed.
The results obtained are depicted in Table 9.
First Sample
m/z
RT
Concentration (ug/ml)
Bisoprolol
326
3.508
0.004
Losartan
423.15
4.725
0.009
amlodipine
409
4.872
0
Atorvastatin
559
6.025
0
candesartan
611.25
6.5
0.005
Second Sample
Bisoprolol
326
3.508
0.002
Losartan
423.15
4.725
0
Amlodipine
409
4.872
0
Atorvastatin
559
6.025
0
Candesartan
611.25
6.5
0.006
Drinking water sample
Bisoprolol
326
3.508
0
Losartan
423.15
4.725
0
Amlodipine
409
4.872
0
Atorvastatin
559
6.025
0
Candesartan
611.25
6.5
0.006
Table 9: Water sample analysis results.
Conclusion
In this paper; a robust, accurate and precise method for the determination and quantification of Bisoprolol, Losartan Amlodipine, Atorvastatin, and Candesartan was developed and validated. The most important feature of the proposed methods was its versatility. The developed method proved to be simple, accurate robust and precise.
Acknowledgements
The authors gratefully acknowledge the support and encouragement from Prof. Mamoon Humaida, Chairman of the Trustees Council of the University of Medical Sciences and Technology (UMST).Khartoum, Sudan.
References
- Oulton RL, Kohn T, Cwiertny DM. Pharmaceuticals and personal care products in effluent matrices: A survey of transformation and removal during wastewater treatment and implications for wastewater management. J Environ Monit JEM. 2010; 12: 1956–1978.
- Ahkola H, Tuominen S, Karlsson S, Perkola N, Huttula T, Saraperä S, et al. Presence of active pharmaceutical ingredients in the continuum of surface and ground water used in drinking water production. Environ Sci Pollut Res Int. 2017; 24: 26778–26791
- Furlong ET, Batt AL, Glassmeyer ST, Noriega MC, Kolpin DW, Mash H, et al. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States: Pharmaceuticals. Sci Total Environ. 2017; 579: 1629–1642.
- Petrie B, Barden R, Kasprzyk-Hordern B. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015; 72: 3–27.
- Asimakopoulos AG, Kannan P, Higgins S, Kannan K. Determination of 89 drugs and other micropollutants in unfiltered wastewater and freshwater by LC-MS/MS: an alternative sample preparation approach. Anal Bioanal Chem. 2017; 409: 6205–6225.
- Chang X, Meyer MT, Liu X, Zhao Q, Chen H, Chen J, et al. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ Pollut Barking Essex 1987. 2010; 158: 1444–1450.
- Dahane S, Gil García MD, Martínez Bueno MJ, Uclés Moreno A, Martínez Galera M, Derdour A. Determination of drugs in river and wastewaters using solid-phase extraction by packed multi-walled carbon nanotubes and liquid chromatography-quadrupole-linear ion trap-mass spectrometry. J Chromatogr A. 2013; 1297: 17–28.
- Mendoza A, Zonja B, Mastroianni N, Negreira N, López de Alda M, Pérez S, et al. Drugs of abuse, cytostatic drugs and iodinated contrast media in tap water from the Madrid region (central Spain):A case study to analyse their occurrence and human health risk characterization. Environ Int. 2016; 86: 107–118.
- Boix C, Ibáñez M, Sancho JV, Rambla J, Aranda JL, Ballester S, et al. Fast determination of 40 drugs in water using large volume direct injection liquid chromatography-tandem mass spectrometry. Talanta. 2015; 131: 719–727.
- Asimakopoulos AG, Kannan P, Higgins S, Kannan K. Determination of 89 drugs and other micropollutants in unfiltered wastewater and freshwater by LC-MS/MS: an alternative sample preparation approach. Anal Bioanal Chem. 2017; 409: 6205–6225.
- Zwiener C, Frimmel FH. LC-MS analysis in the aquatic environment and in water treatment technology--a critical review. Part II: Applications for emerging contaminants and related pollutants, microorganisms and humic acids. Anal Bioanal Chem. 2004; 378: 862–874.
- Borova VL, Maragou NC, Gago-Ferrero P, Pistos C, Thomaidis NS. Highly sensitive determination of 68 psychoactive pharmaceuticals, illicit drugs, and related human metabolites in wastewater by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2014; 406: 4273–4285.
- Wittenberg JB, Simon KA, Wong JW. Targeted Multiresidue Analysis of Veterinary Drugs in Milk-Based Powders Using Liquid Chromatography- Tandem Mass Spectrometry (LC- MS/MS). J Agric Food Chem. 2017; 65: 7288–7293.
- Padrón MET, Afonso-Olivares C, Sosa-Ferrera Z, Santana-Rodríguez JJ. Microextraction techniques coupled to liquid chromatography with mass spectrometry for the determination of organic micropollutants in environmental water samples. Mol Basel Switz. 2014; 19: 10320–10349.
- Dasenaki ME, Thomaidis NS. Multianalyte method for the determination of pharmaceuticals in wastewater samples using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015; 407: 4229–4245.
- Hamrahian SM, Falkner B. Hypertension in Chronic Kidney Disease. Adv Exp Med Biol. 2017; 956: 307–325.
- Nakamura Y, Takagi M, Takeda R, Miyai K, Hasegawa Y. Hypertension is a characteristic complication of X-linked hypophosphatemia. Endocr J. 2017; 64: 283–289.
- Wermelt JA, Schunkert H. Management of arterial hypertension. Herz. 2017; 42: 515–526.
- Research C for DE and. Guidances (Drugs) – Q2 (R1) Validation of Analytical Procedures: Text and Methodology. 2019.