
Research Article
Austin J Anal Pharm Chem. 2019; 6(1): 1114.
Comparative In-vitro Dissolution Study of Some Metronidazole Generic Tablets Under Bio-waiver Conditions by UV-Visible Spectrophotometry
Ahmed EM1, Ibrahim ME1 and Magbool FF2*
1College of Pharmacy, Department of Pharmaceutical Chemistry, Ribat National University, Khartoum, Sudan
2Department of Pharmaceutics and Pharmaceutical Technology, University of Khartoum, Sudan
*Corresponding author: Fatehalrahman F. Magbool, Department of Pharmaceutics and Pharmaceutical Technology, University of Khartoum, Sudan
Received: March 05, 2019; Accepted: April 25, 2019; Published: May 02, 2019
Abstract
This study is aimed to assess the bioequivalence of two generic metronidazole tablets from different manufacturer using in vitro dissolution study under biowaiver conditions by uv-visible spectrophotometry. Dissolution media were USP buffer solutions at pH 1.2 (hydrochloric acid solution), pH 4.5 (acetate buffer solution), and pH 6.8 (phosphate buffer solution). Other general quality assessment tests of these tablets like weight variation, hardness, friability, disintegration time and assay were also determined according to established methods. All brands complied with the official specification for uniformity of weight, friability and disintegration time. Assay of selected tablets revealed that all samples contained over 99% (w/w) of labeled chemical content. The dissolution profiles showed no significant inter brand and intra brand variability. Dissolution results of all the tablet formulations and the innovator brand were further analyzed with difference factor (f1), similarity factor (f2), and dissolution efficiency. These results indicated that both generic metronidazole tablets included in this investigation were bioequivalent with the chosen innovator brand and so may be used interchangeably.
Keywords: Bioequivalence; Dissolution; Disintegration; Metronidazole; Generic; Tablets
Introduction
The process of dissolution plays a vital role in liberation a drug from its dosage form and making it available for subsequent gastrointestinal absorption. Therefore, dissolution analysis of pharmaceutical solid dosage forms is a very important test of product quality and it can be used as a sensitive method for differentiating between formulations of the same therapeutic agent [1,2].
Dissolution of a drug from its dosage form is dependent on many factors, which include not only the physicochemical properties of the drug, but also the formulation of the dosage form and the process of manufacturing [3]. Therefore, constant dissolution analysis of marketed drug products is essential to ensure availability of quality medicines. Metronidazole marketed under the brand name Negazole among others, is an antibiotic and antiprotozoal medication [3]. It is used either alone or with other antibiotics to treat pelvic inflammatory disease, endocarditis, and bacterial vaginosis. It is effective for dracunculiasis, giardiasis, trichomoniasis and amebiasis. It is the drug of choice for a first episode of mild-to-moderate Clostridium difficile colitis [4]. Metronidazole is available by mouth, as a cream, and intravenously [3].
In most of the cases, it would appear that for treatment of above said infections, physicians prescribe metronidazole as a first choice of drug.
Different reports on comparative dissolution study of metronidazole tablets of different countries have been published [5]. No such report is available for metronidazole brands available in Sudan. So the present work was undertaken to evaluate the performance of our local products. Both branded versions and generic products of metronidazole tablets are available in Sudan market but people like to use generic products as they are far cheaper than its branded versions. Generic products can only be substituted with the branded version if they are bioequivalent with the innovator brand. A product is considered bioequivalent with innovator brand when it contains identical amounts of the same active ingredient in the same dose formulation and there is no difference in the availability at the site of drug action when they are administered at the equal molar dose under similar conditions. Bioequivalence studies involve both in-vivo and in-vitro studies. But as bioavailability depends on drug dissolution and the permeability across the gastrointestinal tract in vitro dissolution may be vital in assessing bioequivalence.
In this study, bio- equivalence of three metronidazole brands was assessed in three different dissolution media by in vitro dissolution study. A validated method is essential for the analysis of metronidazole for bio-equivalence study. Several methods that are available for metronidazole analysis are not free from limitation [6]. So first, we chose an economic, rapid uv-visible method for analysis of metronidazole.
Name
Manufacture
Batch number
Production date
Expiry date
Metronidazole (standard)
Azal Pharma Industries CO.LTD-Sudan
3020142
Feb-14
Feb-19
Negazole 500mg (sample (A))
Julphar-Gulf pharmaceutical industries, Ras Al Khaima, U.S.E
155
May-17
May-19
Nilozol 500mg (sample (B))
Blue Nile Pharmaceutical industries - Khartoum
170103
Aug-17
Aug-19
Metrodex 500mg (sample (C))
Consolidated pharmaceutical industries. Khartoum-Sudan
TMF076
May-17
May-19
Table 1: Materials.
Brands
Hardness(Kg/cm)
Weight variation (RSD)
DT(min)
Friability %
Assay %
Sample(A)
12
0.00386
8:27
0.01158
99.88
Sample (B)
12.5
0.0419
2:22
0.1843
98.75
Sample (c)
10.7
0.0243
3:20
0.0184
99.97
Table 2: Summary of the quality control tests results of metronidazole tablets with innovator brand (a).
Figure 1: Structure of metronidazole.
Materials and Methods
Materials
Table 1.
Assay
Absolute drug content: Five pre-weighed tablets from both class tested were crushed; the equivalent weight of a tablet was weighed out and dissolved in 500ml of 0.1M NaOH in a volumetric flask for metronidazole , and filtered. The absorbance reading was determined using uv-visible spectrophotometer at 319nm (95-105%).
Determination of uniformity of weight: Twenty tablets from each of the 3 brands were weighed individually with an analytical weighing balance (Model: AY-200, Shimadzu Corporation, Japan). The average weights for each brand as well as the percentage deviation from the mean value were calculated.
Hardness test: Automatic Tablet Hardness Tester (8M, Dr. Schleuniger, Switzerland) was used to determine the crushing strength. Six tablets were randomly selected from each brand and the pressure at which each tablet crushed was recorded.
Friability test: Twenty tablets of each brand were weighed and subjected to abrasion by employing a Veego friabilator (VFT-2, India) at 25rev/min for 4min. The tablets were then weighed and compared with their initial weights and percentage friability was obtained.
Disintegration test: Six tablets from each brand were employed for the test in distilled water at 37°C using Tablet Disintegration Tester (Model: VDT-2, Veego, India). The time required for disintegrating the tablet and passing completely through the sieve was recorded.
Dissolution test: The dissolution test was undertaken using tablet dissolution tester (TDT-08L, Electrolab, India) in 6 replicates for each brand. Dissolution media were USP buffer solutions at pH 1.2 (hydrochloric acid solution), pH 4.5 (acetate buffer solution), and pH 6.8 (phosphate buffer solution). The medium was maintained at 37±0.5°C. In all the experiments, 5ml of dissolution sample was withdrawn at 0, 5, 10, 15, 30 and 45 min and replaced with equal volume to maintain sink condition. Samples were filtered and assayed by uv-visible method. The concentration of each sample was determined from a calibration curve obtained from pure samples of metronidazole.
Data Analysis
The uniformity of weight was analyzed with simple statistics – percentage deviation while the dissolution profiles were analyzed with difference factor (f1), similarity factor (f2) and some other approaches such as dissolution efficiency.
Results and Discussion
A summary of the results of uniformity of weight, hardness, friability, disintegration and assay are shown in Table 2. Uniformity of weight may serve as a pointer to amount of the active pharmaceutical ingredient (API) contained in the formulation.
All the brands complied with the compendial specification for uniformity of weight, which states that for tablets weighing more than 324mg, not more than 2 tablets should differ from the average weight by 5% or more none will deviate 10% of average weight. Highest deviation was found 2.12% in case of brand C. Hardness is referred to as non-compendial test. The hardness or crushing strength assesses the ability of tablets to withstand handling without fracturing or chipping. It can also influence other parameters such as friability and disintegration. Brand B required the least pressure (94.67N). A force of about 40N is the minimum requirement for a satisfactory tablet 13. Hence, the tablets of all brands were satisfactory for hardness.
Friability test is used to evaluate the tablets resistance to abrasion. Friability is now included in the United States Pharmacopeia [7] as a compendia test. The compendial specification for friability is 1%. Friability for all the brands was below 1%. Disintegration is the process of breaking of tablets in the liquid. Disintegration is a crucial step for immediate release dosage forms because the rate of disintegration affects the dissolution and subsequently the therapeutic efficacy of the medicine. A drug will be released rapidly as the tablet disintegrates. British Pharmacopeia specifies that uncoated tablets should disintegrate within 15min and film coated tablet disintegrate within 30min while USP specification for disintegration is 30min both for uncoated and film coated tablets. All the brands were coated and complied with the both BP and USP specifications for disintegration as maximum DT was found 8:27 min in case of brand A. Potency is the average amount of the active ingredient present per tablet. Brand B contains 98.75% metronidazole (lowest potency). On the other hand brand C contains 99.97% metronidazole (highest potency). All the brands complies both BP and USP specification of as USP specification is that the content of metronidazole should not be less than 90% and more than 110% while BP specifies that the content should not be less than 95% and more than 105%.The results of dissolution studies are graphically represented in Figure 2-4. All dissolution data are based on the actual drug content of the test tablets as calculated from the assay results. Drug release from innovator brand was found a slight higher in all the dissolution media. All the brands released about 80% drug in acidic media (pH 1.2) within 15min. Higher amount of drug was released in acetate buffer medium (pH 4.5) from all the brands. But opposite scenario was observed in case of Phosphate buffer (pH 6.8) drug released was found a slightly lower in this dissolution medium, this is due to the pH depended solubility of metronidazole.
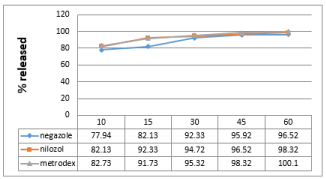
Figure 2: Dissolution profile of Metronidazole in PH (1.2).
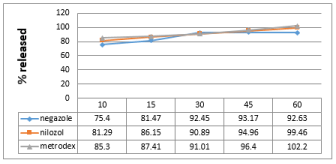
Figure 3: Dissolution profile of metronidazole in PH (4.5).
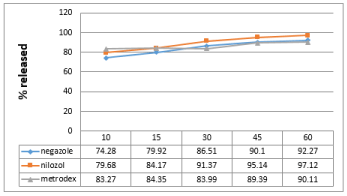
Figure 4: Dissolution profile of metronidazole in PH (6.8).
1.2
4.5
6.8
Samples
F1
F2
F1
F2
F1
F2
sample (B)
4
64
5
66
6
63
sample (C )
5
63
7
58
4
66
Table 3: Calculated difference factor (f1) and similarity factor (f2) of all generic metronidazole tablets.
Samples
1.2
4.5
6.8
AUC
Difference with reference
AUC
Difference with reference
AUC
Difference with reference
Sample (A)
356.37
0
361.96
0
357.84
0
Sample (B)
364.14
7.77
350.14
11.82
355.03
-2.81
Sample (C )
361.02
4.65
345.85
16.11
364.51
6.67
Table 4: Dissolution efficiencies (de) of the two brands of metronidazole tablet with innovator (a).
PH
1.2
4.5
6.8
Brand (B)
102.18%
96.73%
99.22%
Brand (C)
101.30%
95.45%
101.62%
Table 5: Relative dissolution efficiency of metronidazole brands.
Metronidazole is highly soluble at pH 1.2 and 4.5. So, higher dissolution was obtained in these two media. Metronidazole has limited solubility at pH 6.8. So, 90.11% dissolution is justified in case of Phosphate buffer medium (pH 6.8).
Analysis of Dissolution data
Difference factor f1 and similarity factor f2 were calculated by using the following formulas:
f1 = {[3t=1n | Rt - Tt| ]/[3t=1n Rt ]}C…………... (1)
f2 = 50. Log { (1+( 1\n tΣ= 1n(Rt – Tt)2 }-0.5 .100 }………….(2)
where;
n is the number of time points, Rt is the dissolution value of reference product at time t and Tt is the dissolution value for the test product at time t.
Similarity factor f2 has been adopted by FDA and the European Agency for the Evaluation of Medicinal Products (EMEA) by the Committee for Proprietary Medicinal Products (CPMP) as a criterion to compare the similarity of two or more dissolution profiles. Similarity factor f2 is included by the Centre for Drug Evaluation and Research (CDER) in their guidelines such as guidance on dissolution testing of immediate release solid oral dosage forms [7] and guidance on Waiver of In-Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System [8]. Two dissolution profiles to be considered similar and bioequivalent, f1 should be between 0 and 15 while f2 should be between 50 and 100 [7].
Table 3 shows the f1, f2 values of different brands in respect of chosen innovator brand. In f2 calculation, only one measurement is generally considered after the comparator product has reached 85 % dissolution. F1 and f2 values are calculated for dissolution data obtained from the three medium (pH 1.2, 4.5 and 6.8). All the values for f2 are more than 50 and all the f1 values are less than 15. So, we can say that all the brands are equivalent with the innovator band.
Again, dissolution efficiency (DE) was also employed to compare the drug release from various brands. Dissolution efficiency is the area under the dissolution curve within a time range (t1-t2) expressed as a percentage of the dissolution curve at maximum dissolution, over the same time frame [9]. This was calculated from the equation:
where %D is the percentage dissolved at time t, % D(max) is the maximum dissolved at the final time T, and AUC(0-T) is the area under the curve from zero to time T [9].
Table 4 and 5 show the dissolution efficiency of different brands along with the difference with innovator brand. The reference and the test product can be said to be equivalent if the difference between their dissolution efficiencies is within appropriate limits (±10%, which is often used) [9]. DE of all the brands did not differ by 10% with the innovator brand. So, we can say that all the brands are equivalent with the innovator brand.
Conclusion
Our results indicated that all generic metronidazole tablets included in this study seem to have very good bioavailability. All of them comply with BP and USP specifications. They can be considered bioequivalent with the chosen innovator brand. However, in vivo test may be required for final comments regarding the quality of marketed brands of metronidazole.
References
- Hsu H and Ayres JW. Chlorpheniramine Dissolution and Relative Urinary Exerction from Commercial Products. Journal of Pharmaceutical Sciences. 1989; 78: 844-847.
- Bruntion LL. The pharmacological Basis of Therapeutics. Gilman AG, Ra TW, Nies AS and Taylor P (ed), pergamon press. Singapore, Edition 8. 1991: 926-928.
- The American Society of Health-System Pharmacists. 2015.
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical Practice Guidelines for Infection in Adults: Infection Control and Hospital Epidemiology. 2010; 31: 431–455.
- Augsburger LL, Shangraw RF, Giannini RP, Shah VP, Prasad VK and Brown D. Thiazides VIII: Dissolution Survey of Marketed Hydrochlorothiazide Tablets. J Journal of Pharmaceutical Sciences. 1983; 72: 876-881.
- Tripathy KD. Sulfonamides, cotrimoxazole and quinolones. In: Essentials of Medical Pharmacology. Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, India, Edition 5. 2003: 646-652.
- US Food and Drug Administration, Center for Drug Evaluation and Research, 1997: Guidance for industry: Dissolution testing of immediate release solid oral dosage forms.
- US Food and Drug Administration, Center for Drug Evaluation and Research, 2000: Guidance for industry- Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutical classification system.
- Anderson NH, Bauer M, Boussac N, Khan-Malek R, Munden P and Sardaro M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. Journal of Pharmaceutical and Biomedical Analysis. 1998; 17: 811-822.