
Research Article
Austin J Anal Pharm Chem. 2019; 6(1): 1115.
Colurimetric Determination of Olopatadine Hydrochloride Oxidation-Reduction Products in Pure form and Eye Drops
Sebaiy MM*
Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
*Corresponding author: Sebaiy MM, Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
Received: April 14, 2019; Accepted: July 15, 2019; Published: July 22, 2019
Abstract
A novel simple, accurate, sensitive and economical colurimetric method has been established and validated for the determination of the antihistaminic drug, olopatadine hydrochloride in both pure form and eye drops. The spectrophotometric method is divided into four procedures (A, B, C & D). The method is based on the oxidation of the studied drug by a known excess of KMnO4, followed by measuring the decrease in absorption (ΔA) of KMnO4 in 2M H2SO4 (A), in 0.1M NaOH (B) both at 504nm or measuring the increase in absorption of added methyl orange in 0.1M NaOH (C) at 443 nm or measuring the change of KMnO4 into green colour in 1M NaOH (D) at 605nm. The detection limit is reported to be 1.05, 0.62, 0.40 and 0.60μg/ml for procedures A, B, C and D, respectively showing a high degree of sensitivity. The proposed method was successfully validated according to FDA guidelines for the determination of the drug in eye drops with a highly precise recovery and very low relative standard deviation. Finally, the method was compared statistically with a reference method showing equal accuracy, reproducibility and no significant difference with the reported one.
Keywords: Colurimetric; Olopatadine Hydrochloride; KMnO4; Methyl Orange; FDA
Introduction
Olopatadine hydrochloride (Figure 1), is a new antihistaminic drug and chemically, is 11-[3-(dimethylamino)propylidene]- 6,11dihydrodibenzo [b,e] oxepin-2-yl acetic acid hydrochloride [1]. It has a dual selective histamine H1 receptor antagonist and mast cell stabilizer activity showing an excellent anti-allergic activity. This synergic block action of endogenous histamine release leads to a temporary relief of the negative symptoms brought on by histamine. As such, olopatadine is currently used to treat some allergic symptoms like allergic rhinitis, chronic urticaria, eczema, dermatitis and conjunctivitis (itching eyes) [2].
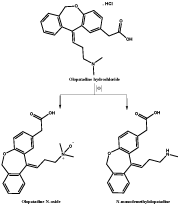
Figure 1: Proposed mechanism for chemical oxidation of olopatadine
hydrochloride into olopatadine N-oxide and N-monodemethylolopatadine.
Literature survey demonstrated that few analytical techniques have been employed for the determination of olopatadine such as spectrophotometry [3-5], derivative spectrophotometry [6,7], high performance liquid chromatography [8-11], high performance liquid chromatography with tandem mass spectrophotometry [12- 14], high performance thin layer chromatography [15,16], capillary eectrophoresis [17] and voltammetry [18].
In comparison to other instrumental analysis methods, spectrophotometric methods are better applied for routine analysis due to their economic, rapid, simple and maintenance free advantages without compromising on accuracy and precision [3].
To the best of our knowledge and comprehensive survey, olopatadine was not determined before spectrophotometrically based on any kind of oxidation-reduction reactions. As such, the present work introduces a simple, reproducible and sensitive spectrophotometric method for the determination of olopatadine relying on oxidation with KMnO4 followed by measuring the decrease in absorption (ΔA) of KMnO4 in acidic medium (A) or basic medium (B) at 504nm or measuring the increase in absorption of added methyl orange in the same basic medium (C) at 443nm or measuring the change of KMnO4 color in higher basic medium concentration at 605nm. This method was then validated to determine of olopatadine hydrochloride in pure form as well as in eye drops to ensure the quality and purity of the sample drug.
Experimental
Apparatus
• Labomed® Spectro UV-VIS Double Beam (UVD-2950) Spectrophotometer with matched 1cm quartz cells and connected to windows compatible computer using UV Win 5 Software v5.0.5.
Materials and reagents
• All solvents and reagents were of analytical grade and double distilled water was used throughout the work.
• Olopatadine HCl was kindly provided by Egyptian Company for Pharmaceutical & Chemical Industries (EIPICO), 10th Of Ramadan City, Egypt. Standard solution of 200μg/ml was prepared by dissolving (0.02g) of the pure drug in 100ml double distilled water.
• Methyl Orange (Aldrich Chemical Co. Ltd., Dorset, England). Standard solution of 3.0x10-3M, was prepared by dissolving (0.096g) of the dye in 100ml double distilled water. The solution was set at room temperature for about two weeks without any significant decay.
• Potassium Permanganate (Aldrich Chemical Co. Ltd., Dorset, England). Standard solution of 5.0x10-3M, was prepared by dissolving (0.079g) of the KMnO4 in 100ml double distilled water and stored in dark bottle [19].
• Sulfuric Acid (El-nasr chemical, Egypt) was prepared as 2M concentration for procedure A.
• Sodium Hydroxide (El-nasr chemical, Egypt) was prepared as two different concentrations of 0.1M for procedures B & C and 1M for procedure D.
• Olohistine® 0.1% eye drops was labeled to contain 1mg/5ml olopatadine HCl (EIPICO, Egypt).
Spectrophotometric procedures
Construction of the calibration curve using procedure A: Aliquot portions of 200μg/ml olopatadine HCl ranging from (0.2- 1.4ml) were transferred into a series of 10ml measuring flasks. To these, 1.5ml of 2M sulfuric acid and 1 ml of 5 x 10-3M potassium permanganate were added then the total volume was adjusted to 10ml with double distilled water. The absorbance of a reagent blank (similarly prepared without the drug) was measured against each drug concentration at 504nm.
Construction of the calibration curve using procedure B: Aliquot portions of 200μg/ml olopatadine HCl ranging from (0.2- 1.2ml) were transferred into a series of 10ml measuring flasks. To these, 0.5ml of 0.1M sodium hydroxide and 1.5ml of 5x10-3M potassium permanganate were added then the total volume was adjusted to 10ml with double distilled water. The absorbance of a reagent blank was measured against each drug concentration at 504nm.
Construction of the calibration curve using procedure C: Aliquot portions of 200μg/ml olopatadine HCl ranging from (0.8- 2.2ml) were transferred into a series of 10ml measuring flasks. To these, 0.5ml of 0.1M sodium hydroxide, 1.5ml of 5 x 10-3M potassium permanganate and 1ml of 3x10-3M methyl orange were added and the flasks were shaken and allowed to stand for 5 minutes at room temperature. The total volume was then adjusted to 10ml with double distilled water and absorbance of methyl orange was measured at 443nm, against a reagent blank.
Construction of the calibration curve using procedure D: Aliquot portions of 200μg/ml olopatadine HCl ranging from (0.2- 1.4ml) were transferred into a series of 10ml measuring flasks. To these, 2ml of 1M sodium hydroxide and 3ml of 5x10-3M potassium permanganate were added then the total volume was adjusted to 10ml with double distilled water. After 25 minutes, the absorbance of each drug concentration was measured at 605nm against reagent blank.
Procedure for pharmaceutical preparation
For Olohistine® eye drops: An accurately volume of 1.8ml of the eye drops (olopatadine 1mg/5ml) was transferred to a 10ml measuring flask and completed to volume with double distilled water to give an equivalent final concentration of 200μg/ml. The procedures A, B, C & D were then conducted as mentioned above under the general procedures applying standard addition techniques.
Results and Discussion
Chemistry of the oxidation product
The proposed method is based on the oxidation of olopatadine hydrochloride with potassium permenganate. Figure 1 is depicting the proposed scheme of olopatadine hydrochloride oxidation into olopatadine N-oxide and N-monodemethylolopatadine. Both compounds are expected to be the major oxidation products similar to the pathway of the cited drug metabolism in the body as reported by Jiro K. et al. [20].
Optimization of the reaction conditions
The optimum conditions for the method development were established by varying each specific parameter and keeping the others constant and observing the effect produced on the absorbance of the colored species. The optimum parameters are reported in Table 1.
Absorption spectra: Absorption spectra of olopatadine HCl with the reagents were studied over a range of 400-800nm. Potassium permanganate reacts with olopatadine HCl in 2M acidic medium (A), or in 0.1M basic medium (B) and the decrease in absorption can be measured at 504nm. Also, the increase in absorption of added methyl orange in the same basic medium (C) can be measured at 443nm or the change of KMnO4 color into green in 1M basic medium (D) can be measured at 605nm as shown in Figure 2.
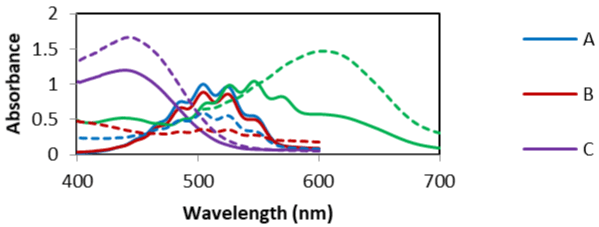
Figure 2: Absorption spectra of potassium permenganate in 2M H2SO4 (A),
in 0.1M NaOH (B), with methyl orange in 0.1M NaOH (C) & in 1M NaOH (D)
____ and the effect of olopatadine HCl (20μg/ml) addition on each spectrum
------- at 504nm (A & B), 443nm (C) & 605nm (D), respectively.
parameters
Procedure A
Procedure B
Procedure C
Procedure D
λmax
504nm
504nm
443nm
605nm
Volume of the media
1.5ml 2M H2SO4
0.5ml 0.1M NaOH
0.5ml 0.1M NaOH
2ml 1M NaOH
Volume of the reagents
1ml KMnO4
1.5ml KMnO4
1.5ml KMnO4 + 1ml methyl orange
3ml KMnO4
Time of reaction between olopatadine and KMnO4
immediate
25 minutes
Time after dye addition
------
------
5 minutes
------
Temperature
Ambient
Table 1: Analytical parameters and spectrophotometric characteristics of the proposed method for olopatadine hydrochloride determination.
Effect of temperature: Effect of temperature was studied and results showed that there is no an evident effect of temperature on the reaction as increase in temperature is not accompanied with any increase in absorbance and so, optimum reaction was performed at room temperature.
Effect of addition sequence: Addition sequences were studied and results revealed that the most appropriate sequence was the drug then the added acid or base then the added potassium permenganate (procedures A, B & D) and finally the dye addition (procedure C).
Effect of time: The effect of time on the oxidation reaction was studied to obtain the highest and most stable absorbance. This absorbance can be achieved immediately after the reaction between the drug and potassium permenganate (procedures A&B) or after 25 minutes (procedure D) in higher basic concentration while the reaction between potassium permenganate and methyl orange was reported to be stable after 5 minutes (procedure C).
Effect of acidity and basicity: To study the effect of sulfuric acid and sodium hydroxide volumes, the reaction was performed in a series of 10ml volumetric flasks containing different volumes (0.5- 3.5ml) of 2M H2SO4, 0.1M NaOH, or 1M NaOH, separately. It was found that the maximum absorbance was obtained when using 1.5ml of 2M H2SO4 (procedure A), 0.5ml of 0.1M NaOH (procedures B&C) or 2ml 1M NaOH (procedure D).
Effect of permenganate concentration: When studying the effect of potassium permanganate concentration referring to decrease of its color intensity (procedures A&B) or increase of methyl orange color intensity (procedure C) or change in KMnO4 colour (procedure D), it was observed that the absorbance reached its maximum when 1ml of 5x10-3M potassium permanganate was used in case of procedure A, 1.5ml was sufficient for procedures B&C, while 3ml was optimum for procedure D.
Effect of dye concentration: In order to ensure a linear relationship between the different concentrations of olopatadine hydrochloride and the increase in absorbance of methyl orange (procedure C), experiments were performed in 0.5ml of 0.1M sodium hydroxide, 1.5ml of 5.0 x10-3M potassium permanganate and different volumes of methyl orange. It was found that 1.0ml of 3.0 x10-3M methyl orange was enough to give a maximum absorbance at 443nm.
Method validation
The method validation was performed according to food and drug administration and international conference of harmonization guidelines (ICH) [21].
Linearity: Five different concentrations of olopatadine HCl for each procedure was prepared for linearity studies. The linearity ranges of absorbance as a function of drug concentration (Table 2) provided acceptable indication about sensitivity of reagents used. Linear regression equations of procedures A, B, C and D were found to be y = 0.013x + 0.044, y = 0.0204x + 0.0098, y = 0.0115x - 0.0885, and y = 0.0349x + 0.126, respectively and the regression coefficient values (R2) were found to be 0.9995, 1, 0.9992 and 0.9996, respectively indicating a high degree of linearity for all procedures (Figure 3).
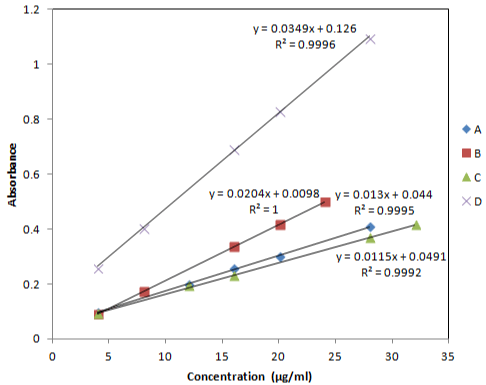
Figure 3: Calibration curves of olopatadine HCl with potassium permenganate
in 2M H2SO4 (A), in 0.1M NaOH (B), with methyl orange in 0.1M NaOH (C)
& in 1M NaOH (D).
parameters
Procedure A
Procedure B
Procedure C
Procedure D
Taken μg/ml
Found μg/ml
Recovery %
Taken μg/ml
Taken μg/ml
Taken μg/ml
Taken μg/ml
Found μg/ml
Recovery %
Taken μg/ml
Found μg/ml
Recovery %
4
3.92
98.99
4
3.99
99.75
16
16.27
101.7
4
3.94
98.5
12
12
100
8
8.02
100.36
24
24.43
101.81
8
8.14
101.78
16
16.3
101.92
16
16.1
100.67
28
27.82
99.78
16
16.31
101.96
20
19.76
98.91
20
20.04
100.24
40
39.82
99.56
20
20.28
101.42
28
28
100
24
24.08
100.36
44
43.73
99.71
28
27.85
99.48
Mean
99.76
100.28
100.37
100.63
±SD
1.454
0.336
1.266
1.54
±SE
0.65
0.15
0.56
0.69
±RSD
1.457
0.335
1.261
1.53
Variance
2.11
0.11
1.6
2.38
Slope
0.013
0.02
0.011
0.034
LOD
1.05
0.62
0.4
0.6
LOQ
3.51
2.08
1.34
2.01
Table 2: Results of the analysis for determination of olopatadine hydrochloride sample using the proposed method.
Accuracy: The accuracy of the method was determined by investigating the recovery of olopatadine HCl concentration levels covering the specified range using the standard addition technique. It was performed by adding a fixed standard drug concentration at different levels of the eye drops solution and the proposed method was followed. From the amount of the drug estimated, the percentage recovery was calculated and the results are shown in Table 3.
parameters
Procedure A
Procedure B
Procedure C
Procedure D
Added pure Olo μg/ml
Taken μg/ml
Found μg/ml
Recovery %
Added pure Olo μg/ml
Taken μg/ml
Found μg/ml
Recovery %
Added pure Olo μg/ml
Taken μg/ml
Found μg/ml
Recovery %
Added pure Olo μg/ml
Taken μg/ml
Found μg/ml
Recovery %
4
0
4.07
101.92
4
0
4.06
101.6
4
12
16.27
101.7
4
0
4.05
101.49
4
8
12
100
4
4
8.07
100.98
4
20
24.26
101.08
4
4
7.97
99.62
4
16
20.23
101.15
4
16
19.7
98.52
4
24
28
100
4
12
15.88
99.25
4
20
24.46
101.92
4
20
24.13
100.57
4
36
39.82
99.56
4
16
19.61
98.05
4
24
28.07
100.27
4
24
27.53
98.34
4
40
44.08
100.19
4
24
28.41
101.49
Mean
101.05
100.01
100.51
99.98
±SD
0.9
1.48
0.87
1.49
±SE
0.4
0.66
0.38
0.66
±RSD
0.89
1.48
0.86
1.49
Variance
0.8
2.19
0.75
2.22
Table 3: Application of standard addition technique for the determination of Olohistine® eye drops using the proposed method.
Precision: Intraday precision and interday reproducibility were evaluated by calculating relative standard deviations and recoveries of three replicate determinations using three different concentrations of olopatadine HCl. It was found that the reproducibility of the method in the basic medium (%RSD ≈ 1.9) is much better than in the acidic medium (%RSD ≈ 2.3). However, the results obtained by the proposed method were found to be acceptable.
Specificity: The specificity studies revealed that the presence of the excipents in the eye drops formulation didn’t show any kind of impurity interference, since the recoveries lied in the range of 98- 102% as reported in Table 3.
Limits of detection and limits of quantification: The calculation of limits of detection and quantitation was based on the following equations: LOD = 3.3 S/K and LOQ = 10 S/K, respectively, where S is the standard deviation of the seven replicate values under the same conditions as for the sample analysis in the absence of analyte and K is the sensitivity, namely, the slope of calibration graph. Limits of detection in case of procedures A, B, C and D were calculated to be 1.05, 0.62, 0.40 and 0.60μg/ml and limits of quantification were 3.51, 2.08, 1.34 and 2.01μg/ml, respectively (Table 2).
Robustness: The robustness of the method was evaluated by making small changes (± 0.05ml) in the volume of H2SO4, NaOH, methyl orange and KMnO4 keeping the other conditions constant where the effect of the changes was studied on the percent recovery and standard deviation of 20μg/ml olopatadine HCl. The changes had negligible influence on the results where SD values were in the acceptable range (= 1.93) as reported in Table 4.
Procedure / Parameter changes
Mean recovery ± SD
CV (%)
% Accuracy
A + 0.05ml H2SO4
100.53 ± 1.83
1.8
0.53
A - 0.05ml H2SO4
99.38 ± 1.93
1.95
-0.62
B + 0.05ml NaOH
99.44 ± 1.92
1.93
-0.56
B - 0.05ml NaOH
99.93 ± 0.85
0.86
-0.07
C + 0.05ml Methyl orange
99.64 ± 1.27
1.28
-0.36
C - 0.05ml Methyl orange
100.73 ± 1.88
1.87
0.73
D + 0.05ml KMnO4
101.32 ± 1.68
1.65
1.32
D - 0.05ml KMnO4
100.33 ± 1.93
1.92
0.33
Table 4: Results of the robustness for 20 μg/ml olopatadine hydrochloride sample using the proposed method.
Ruggedness: The ruggedness of the method was tested by measuring three concentrations of the standard working solution using a different double beam spectrophotometer (model Jenway 6500, UK). The absorbances in case of the four procedures for both instruments were exactly similar indicating that the method is fairly rugged.
According to FDA-ICH guidelines, the obtained values indicated high sensitivity of the proposed method.
Statistical analysis of the pharmaceutical formulation
Olohistine→ eye drops has been successfully analyzed by the proposed method. Results obtained were compared to those obtained by applying reference method [7] where Student’s t-test and F-test were performed for comparison. Results are shown in table 5 where the calculated t and F values were less than tabulated values at p= 0.05, which in turn indicate that there is no significant difference between proposed method and reference one relative to precision and accuracy.
Parameters
Procedure A
Procedure B
Procedure C
Procedure D
Reported method [7]
N
5
5
5
5
4
Mean Recovery
101.05
100.01
100.51
99.98
100.56
SE
0.36
0.33
0.28
0.66
0.09
Variance
1.01
0.69
0.58
2.22
4.43
Student-t**
0.69 (1.89)a
0.61 (1.89)a
0.09 (1.89)a
0.63 (1.89)a
F-test**
1.85 (6.59)b
1.46 (6.59)b
1.99 (6.59)b
1.49 (6.59)b
a and b are the theoretical Student t-values and F-ratio at p= 0.05.
Table 5: Statistical analysis of results obtained by the proposed method applied on Olohistine® eye drops compared with reference method.
Conclusion
Unlike GC and HPLC techniques, spectrophotometry is simple and inexpensive. The proposed method requires reagents which are very cheap and readily available, no pH adjustment is required and the procedures do not involve any critical reaction conditions or tedious sample preparation. According to FDA guidlines, the method is simple, fast, accurate, sensitive, rugged and free from interference by common additives and excipients which makes it ideal for routine quality control analysis. The amounts obtained by the proposed method for the eye drops lied in the acceptable range of 98-102% and were statistically superior to the reference method with respect to both sensitivity and selectivity.
References
- Ohshima E, Ohtaki S, Sato H, Kumazawa T, Obase H, Ishii A, et al. Synthesis and antiallergic activity of 11-(aminoalkylidene)-6,11-dihydrodibenz [b,e] oxepin derivatives. J Med Chem. 1992; 35: 2074-2084.
- James M. A review of the use of olopatadine in allergic conjunctivitis. Int Ophth. 2004; 25: 171-179.
- Suddhasattya D, Vikram R, Swetha B, Sandeep K, Sudhir S, Dhiraj K, et al. Method development and validation for the estimation of olopatadine in bulk and pharmaceutical dosage forms and its stress degradation studies using UV-VIS spectrophotometric method. Int J Pharm Pharm Sci. 2010; 2: 212-218.
- Annapurna M, Bindu G, Divya I. New analytical methods for the determination of olopatadine (an anti-allergic drug) in eye drops. Drug Inv Tod. 2012; 4: 441-443.
- Jain D, Basniwal P. Spectrophotometric determination of olopatadine hydrochloride in eye drops and tablets. J Pharm Res. 2013; 12: 48-52.
- Annapurna M, Bindu G, Divya I. Derivative spectrophotometric methods for the determination of Olopatadine in pharmaceutical dosage form. Drug Inv Tod. 2012; 4: 540-542.
- Rele V. UV-Spectrophotometric Estimation of Olopatadine hydrochloride in Bulk and Pharmaceutical Dosage Form by area under Curve and second Order Derivative Methods. Res J Pharm Tech. 2015; 8: 265-269.
- Rele R, Warkar B. Application of high performance liquid chromatographic technique for olopatadine hydrochloride and its impurity in ophthalmic solution. Int J Chem Sci. 2011; 9: 601-614.
- Parth B, Jawed A. Development and validation of stability indicating RPHPLC method for estimation of olopatadine hydrochloride in bulk drug and its formulations. Int J Pharm Sci Rev Res. 2011; 9: 153-158.
- Pawan B, Deepti J. ICH guideline practice: application of novel RP-HPLCDAD method for determination of olopatadine hydrochloride in pharmaceutical products. J Anal Sci Tech. 2013; 4: 1-6.
- Maksic J, Jovanovic M, Rakic T, Popovic I, Ivanovic D, Jancic-Stojanovic B. Chromatographic analysis of olopatadine in hydrophilic interaction liquid chromatography. J Chromatogr Sci. 2015; 53: 680-686.
- Fujita K, Magara H, Kobayashi H. Determination of olopatadine, a new antiallergic agent, and its metabolites in human plasma by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 1999; 731: 345-352.
- Koichiro F, Xiao-Pen L, Takeshi K, Junichi S, Keizo S. Determination of some antiallergic drugs in human plasma by direct-injection high-performance liquid chromatography-tandem mass spectrometry. Forens Tox. 2006; 24: 8-16.
- Zhu P, Wen G, Fan P, Zhou L, Fan X, Chen M, et al. A rapid and sensitive liquid chromatography-tandem mass spectrometry method for determination of olopatadine concentration in human plasma. J Anal Toxicol. 2011; 35: 113- 118.
- Mahajan A, Gandhi P, Pandita N, Gandhi S, Deshpande P. Validated high performance thin layer chromatographic method for estimation of olopatadine hydrochloride as bulk drug and in ophthalmic solutions. Int J Chem Tech Res. 2010; 2: 1372-1375.
- Varghese J, Kumar M, Ravi K. Stability-indicating high-performance column liquid chromatography and high-performance thin-layer chromatography methods for the determination of olopatadine hydrochloride in tablet dosage form. J AOAC Int. 2011; 94: 1815-1820.
- Güray T, Turan T, Tunçel M, Uysal D. A Validated Capillary Electrophoretic Method for the Determination of Olopatadine and Its Application to a Pharmaceutical Preparation of Eye Drops. J AOAC Int. 2017; 100: 206-211.
- Sreedhar Y, Sreenivasulu A, Sunil M, Nagaraju M. Voltammetric Determination of Olopatadine Hydrochloride In Bulk Drug Form And Pharmaceutical Formulations. Int J Pharm Sci Res. 2012; 3: 2320-2324.
- Mendham J, Denny R, Barnes J, Thomas K. “Vogel Qualitative analysis” Pearson Education Limited. Seventh edition. 2000.
- Jiro K, Keiko I, Eiichi F, Takashi K, Hiroyuki K. Effects of Olopatadine, a New Antiallergic Agent, on Human Liver Microsomal Cytochrome P450 Activities. Drug Metab Dispos. 2002; 30: 1504-1511.
- Guidance for Industry: Q2B Validation of Analytical Procedures: Methodology. International Conference of Harmonization (ICH). 1996.