
Research Article
Austin J Anal Pharm Chem. 2019; 6(2): 1116.
Synthesis and Evaluation of Antimicrobial Properties of Some Novel Indole Pyridine Based Chalcones
Looga AM1, Ambassa P2*, Kamga J2, Hortense GK3, Ngadjui BT2 and Ngameni B1*
¹Department of Pharmacognosy and Pharmaceutical Chemistry, University of Yaoundé I, Yaoundé, Cameroon
²Department of Organic Chemistry, University of Yaoundé I, Yaoundé, Cameroon
³Department of Microbiology, parasitology, hematology and infectious diseases, University of Yaoundé I, Yaoundé, Cameroon
*Corresponding author: Ambassa P, Department of Organic Chemistry, University of Yaoundé I, Yaoundé, Cameroon, Email: ambassapantaleon@yahoo.fr
Ngameni B, Department of Pharmacognosy and Pharmaceutical Chemistry, University of Yaoundé I, Yaoundé, Cameroon, Email: bath_ngameni@yahoo.fr
Received: April 22, 2019; Accepted: July 15, 2019; Published: July 22, 2019
Abstract
A series of novel substituted Indolylchalcone derivatives 2a-d were synthesized via Claisen-Schmidt condensation between some selected indolic aldehyde and pyridynic acetophenone. The structures of the products were elucidated by spectroscopic analysis (1H and 13C NMR). All compounds were screened for their antibacterial and antifungal activity against six different bacterial strains such as Escherichia coli ATCC 25922, Klebsiella pneumonia ATCC 700603, Staphylococcus aureus ATCC 25923, Proteus mirabilis, Salmonella typhi and Pseudomonas aeruginosa and against one fungal strain Candida albicans. The Results reveal that all compounds exhibited moderate to good antibacterial and antifungal activities.
Keywords: Synthesis; Chalcone; Indole; Pyridine; Antibacterial & Antifungal Activity
Introduction
Chalcones or 1,3-diphenyl-2-propen-1-one derivatives are a class of open chain flavonoids in which two aromatic rings are linked by a three carbon a,β-unsaturated carbonyl skeleton. Chalcone are an important class of natural products which exhibit interesting pharmacological activities [1]. Some of the chalcones have been reported to possess many biological properties such as antimicrobial [2-4], anticancer [5], antimalarial [6], anti-inflammatory [7], antituberculosis [8], antioxidant [9,10], analgesic [11], anticonvulsant [12] and antidiabetic [13].
A good safety profile, possibility of oral administration [14] and easy synthesis are the major factors contributing to the increasing interest in exploring the pharmacological activities of chalcones. During the last decade, the antimicrobial resistant have represented the major problem facing the world, so that several new antibiotics and antifungal agents are accepted each year to help for the treatment of infectious diseases.
Indole derivatives have recently been reported to have potent antibacterial and antifungal property against human pathogens resistant to classic antibiotic [15]. In the other hand pyridine derivatives have been reported for variety of biological activities and number of compounds are in clinical uses [16]. Moreover some of those derivatives possess good antibacterial property against staphylococcus epidermis, streptococcus mutans and streptococcus sanguinis and are only slightly cytotoxic [17].
In our continuing effort to discover new antimicrobial agents [4] with excellent therapeutic index, we carry out the present research in order to combine the antimicrobial effect of chalcone moiety with those of indole and pyridine derivative. Hence, this research illustrated the synthesis of novel Indole pyridine chalcone derivatives and their biological screening against some gram positive and gram negative bacterial species including fungus specie by standard methods.
Experimental
Chemistry
All starting materials and solvents were purchased from Sigma- Aldrich and Alfa Aesar and used without further purification. Purity of the compounds was checked on silica gel G TLC plates of 2mm thickness using n-hexane and ethyl acetate as solvent system. The visualization of spot was carried out under UV light. NMR study was performed on Brucker AMX-300 using DMSO-d6 as solvent at 300MHz. Tetramethylsilane (TMS) was used as internal reference and chemical shifts are expressed in d ppm.
The key method of our synthesis involves the Claisen-Schmidt condensation of equimolar quantities of a substituted acetophenone with substituted aldehydes in the presence of anhydrous alcoholic alkali.
The various derivatives were synthesized according to procedure described by [18].
Synthesis of (E)-3-(1H-indol-3-yl)-1-(pyridin-3-yl) prop-2- en-1-one (2a): 0.173mL of 3-acetylpyridine (1.58mmol) was added dropwise with stirring to a solution of indole-3-carbaldehyde (153.87mg, 1.06mmol) in anhydrous ethanol (15mL). The mixture was treated with piperidine (0.155mL, 1.58mmol) and refluxed for 28 h. Upon completion, the mixture was cooled by pouring crushed ice in it to complete the crystallization. The solid was filtered by Buchner filtration and washed with ice-cold MeOH (30mL) and dried at room temperature for 24-48 h to yield 21.67% of a bright yellow powder
1HNMR (300 MHz; DMSO-d6) dppm (m, J(Hz)): 11.98 (1H; .s; H-3’), 9.29 (1H; d; J=3Hz; H-2), 8.80 (1H; dd; J=3Hz et 6Hz; H-4), 8.45 (1H; dd; J=3Hz and 6Hz; H-6), 8.17 (1H; s; H-2’), 8.14 (1H; m; H-5), 8.11 (1H; d; J=15Hz; H-β), 7.65 (1H; d; J=15Hz; H-a), 7.60 (1H; dd; J=3Hz and 6Hz; H-8’), 7.49 (1H; m; H-5’), 7.26 (1H; m; H-6’), 7.24 (1H; m; H-7’). 13CNMR (75 MHz; DMSO-d6) dppm (m, J(Hz)): 187.9 (-C=O), 152.7 (C-4), 149.3 ( C-2), 139.9 ( C-β), 137.6 ( C-4’), 135.5 ( C-6), 134.0 ( C-1), 133.7 (C-2’), 125.1 (C-9’), 123.9 (C- a), 122.8 (C-5), 121.3 (C-6’), 120.6 ( C-7’), 115.0 ( C-8’), 112.9 ( 2C; C-1’ and C-5’).
Synthesis of (E)-3-(1-methyl-1H-indol-3-yl)-1-(pyridin-3-yl) prop-2-en-1-one (2b): 0.173mL of 3-acetylpyridine (1.58mmol) was added dropwise with stirring to a solution of N-methylindole-3- carbaldehyde (168.54mg, 1.06mmol) in anhydrous ethanol (15mL). The mixture was treated with piperidine (0.155mL, 1.58mmol) and refluxed for 28 h. Upon completion, the mixture was cooled by pouring crushed ice in it to complete the crystallization. The solid was filtered by Buchner filtration and purified by column chromatography using silica gel as adsorbent and a mixture of hexane and ethyl acetate as eluent. After recrystallization in 10% Hexane-ethyl acetate we obtained (2b) as a yellow powder with 67.33% yield.
1HNMR (300 MHz; DMSO-d6) dppm (m, J(Hz): 9.29 (1H; d; J=3Hz; H-2), 8.81 (1H; dd; J=3Hz and 6Hz; H-4), 8.46 (1H; dd; J=3Hz and 6Hz; H-6), 8.17 (1H; s; H-2’), 8.16 (1H; dd; J=3Hz and 6Hz; H-8’), 8.07 (1H; d; J=15Hz; H-β), 7.65 (1H; d; J=15Hz; H-a), 7.59 (1H; m; H-5), 7.58 (1H; m; H-5’), 7.34 (1H; m; H-6’), 7.32 (1H; m; H7’), 3.88 (3H; s; Me-N). 13CNMR (75MHz; DMSO) dppm: 187.7 (C=O), 152.6 ( C-4), 142.2 (C-3), 139.1 (C-β), 138.0 (C-4’), 137.2 ( C-2’), 135.6 (C- 6), 133.6 ( C-1), 125.5 (C-9’), 123.8 (C-5), 122.8 ( C-6’), 121.5 (C-7’), 120.7 ( C-8’), 115.0 (C-a), 111.8 (C-1’), 110.8 (C-5’), 33.1(Me).
Synthesis of (E)-3-(1-methyl-1H-indol-3-yl)-1-(pyridin-4-yl) prop-2-en-1-one (2c): 0.173mL of 4-acétylpyridine (1.58mmol) was added dropwise with stirring to a solution of N-methylindole-3- carbaldehyde (168.54mg, 1.06mmol) in anhydrous ethanol (15mL). The mixture was treated with piperidine (0.155mL, 1.58mmol) and refluxed for 28 h. Upon completion, the mixture was cooled by pouring crushed ice in it to complete the crystallization. The solid was filtered by Buchner filtration and purified by column chromatography using silica gel as adsorbent and a mixture of hexane and ethyl acetate as eluent. After recrystallization in 10% Hexane-ethyl acetate we obtained (2c) as a yellow powder with 31.32% yield.
1HNMR (300 MHz; DMSO-d6) dppm (m, J(Hz): 8.85 (2H; d; J=6Hz; H-3 and H-5), 8.17 (1H; s; H-2’), 8.15 (1H; dd; J=3Hz and 6Hz; H-8’), 8.07 (1H; d; J=15Hz; H-β), 7.99 (2H; d; J=6Hz; H-2 and H-6), 7.58 (1H; m; H-5’), 7.57 (1H; d; J=15Hz; H-a), 7.34 (1H; m; H-6’), 7.32 (1H; m; H-7’), 3.88 (3H; s; N-Me). 13CNMR (75 MHz; DMSO) dppm: 188.3 ( C=O), 150.6 (2C; C-3 and C-5), 144.6 (C-1), 140.1 ( C-β), 138.1 ( C-4’), 137.7 ( C-2’), 125.5 ( C-9’), 122.9 (C-6’), 121.8 (C-7’), 121.7 (2C; C-2 and C-6), 120.7 ( C-8’), 114.6 (C-a), 111.8 ( C-1’), 110.9 (C-5’), 33.1 ( Me-N).
Synthesis of (E)-3-(1H-indol-3-yl)-1-(pyridin-4-yl) prop- 2-en-1-one (2d): 0.173mL of 4-acetylpyridine (1.58mmol) was added dropwise with stirring to a solution of indole-3-carbaldehyde (153.87mg, 1.06mmol) in anhydrous ethanol (15mL). The mixture was treated with piperidine (0.155mL, 1.58mmol) and refluxed for 28 h. Upon completion, the mixture was cooled by pouring crushed ice in it to complete the crystallization. The solid was filtered by Buchner filtration and purified by column chromatography using silica gel as adsorbent and a mixture of hexane and ethyl acetate as eluent. After recrystallization in 10% Hexane-ethyl acetate we obtained (2d) as a yellow powder with 25.18% yield.
1HNMR (300 MHz; DMSO-d6) dppm(m, J(Hz): 12.03 (1H; s; H-3’), 8.82 (2H; d; J=6Hz; H-3 and H-5), 8.18 (1H; s; H-2’), 8.11 (1H; d; J=15Hz; H-β), 8.10 (1H; m; H-8’), 7.77 (2H; d; J=6Hz; H-2 and H-6), 7.58 (1H; d; J=15Hz, H-a), 7.51 (1H; m; H-7’), 7.27 (1H; m; H-5’), 7.23 (1H; m; H-6’).
Biology
Paper disc diffusion technique
Anti-bacterial and anti-fungal activities of synthesized compounds were assessed by paper disc diffusion method [19,20]. Here, all the synthesized compounds’ antimicrobial activities were calculated from the zone of inhibition in Mueller-Hinton (anti-bacterial) and Sabouraud dextrose culture media (antifungal). First, the aforesaid sterilised (120°C for 30 min in autoclave) culture medium (40-50°C) was prepared and poured into a Petri dish (3-4mm), solidified and seeded with different micro-organisms (Escherichia coli (ATCC 25922), Klebsiella pneumonia (ATCC 700603), Staphylococcus aureus(ATCC 25923), Proteus mirabilis, Salmonella typhi and Pseudomonas aeruginosa and Candida albicans) in on each plate. Simultaneously, different dilutions of synthesized compounds and standard solutions were prepared in dimethylsulfoxide (DMSO). To identify the anti-microbial activities, the paper impregnated with synthesized compounds were placed on the solidified Mueller- Hinton/Sabouraud dextrose medium and incubated at 37°C for 24 hours.
In addition to that, Norfloxacin (100mg/disc) and Nystatin (100mg/disc) were also placed on the Petri dish and had been considered as a standard for antibacterial and antifungal activities, respectively. The solvent control was charged separately on a Petri dish. For each compound, anti-microbial activity has been measured from its zone of inhibition against standard drugs.
Minimum inhibitory concentration
Minimum Inhibitory Concentration (MIC) is the least concentration of an antimicrobial agent having the potency to inhibit the detectable growth of a microorganism after an overnight period of incubation in appropriate culture media [20,21]. It is used to detect the potency and drug resistance nature of anti-microbial agents. A highly potent anti-microbial agent had the least value of MIC and needed less quantity to inhibit the visible growth of the microorganism. An anti-microbial agent that is called as less active needs high concentration to kill the micro-organism and to have greater MIC value. Here, the anti-microbial activities of different concentrations of the synthesized compounds in dimethylsulfoxide (DMSO) were calculated through MIC value.
Each concentration of the synthesized compounds (1000, 500, 250, 125, 62.5 and 31.25μg/mL) was tested with bacteria/fungal seeded culture medium (Mueller-Hinton and Sabouraud dextrose) for 24 hours/48 hours at 37°C. The different microbial inoculums were used to prepare compared Mc Farland 0.5 standard bacteria suspensions.
The minimal inhibitory concentration was read by observing the opacity of the wells. The inhibition of microbial growth by the test compounds left some wells clear (not turbid), except in the negative control. The least concentrated well in which no turbidity was observed was considered the minimum inhibitory concentration of the test compound.
Result and Discussion
Chemistry
The entire synthesized compounds were prepared from the mentioned synthetic scheme that is depicted in Scheme 1. The novel bi-heterocyclic chalcone derivatives were prepared by a Claisen- Schmidt condensation reaction between substituted acetophenone derivatives and 2 substituted 1H-indole-3-carbaldehyde.
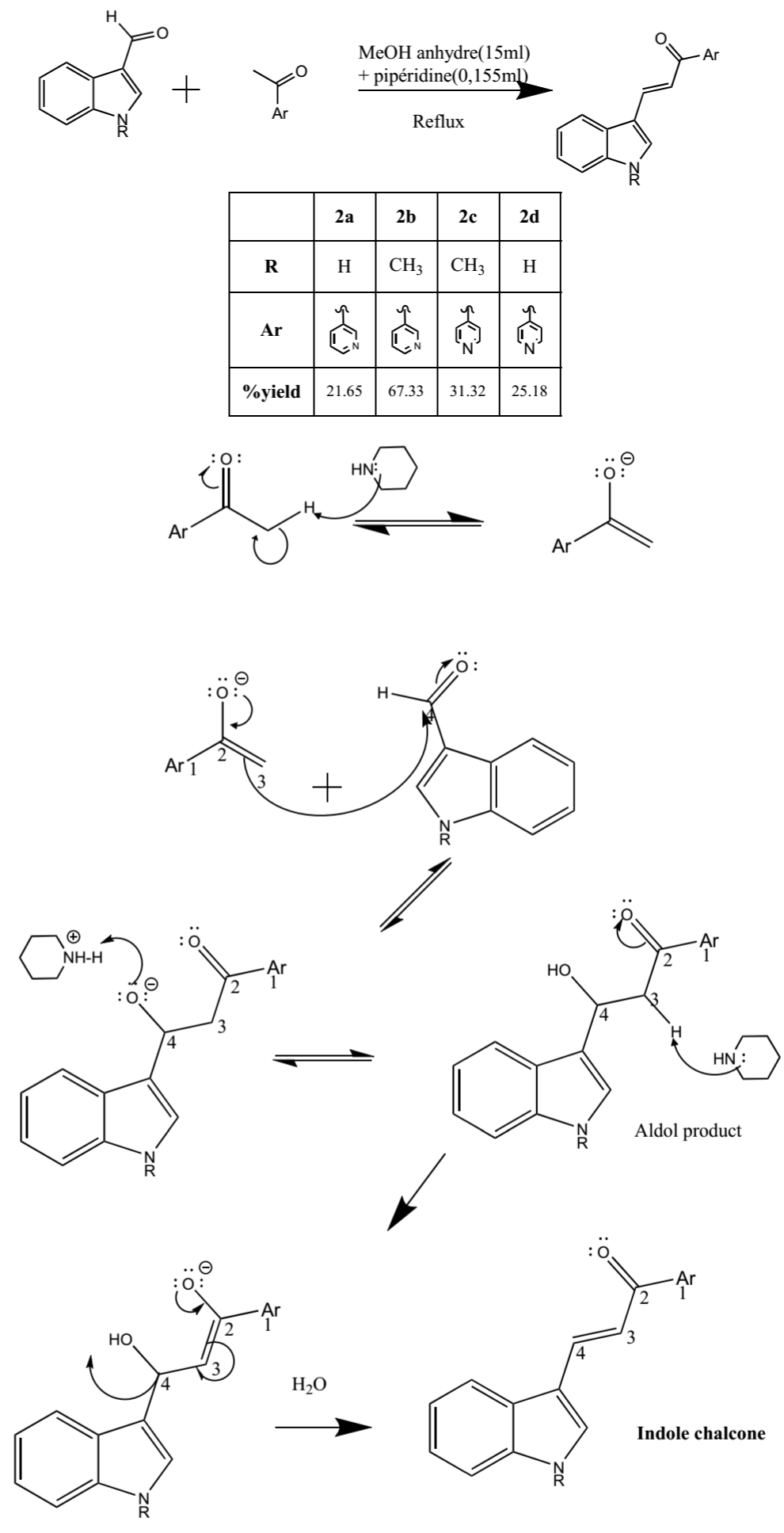
Scheme : General reaction and mechanism of chalcone synthesis.
The chemical structure of all our synthesized compounds was elucidated by 1HNMR, 13CNMR, and elemental analysis. The number of protons and carbons present in the synthesized compounds was identified by 1HNMR and 13CNMR spectroscopy, respectively from the chemical shift (d in ppm). The proton spectra showed a doublet at d 7.60 and 8.11ppm corresponding to the a and β hydrogen respectively of the unsaturated carbon with a coupling constant of 15Hz which confirmed their trans stereochemical configuration; a doublet at d 7.77-8.82ppm corresponding to aromatic protons (Ar-H); a doublet of doublet at d 8.80-8.81ppm corresponding to aromatic protons (Ar-H); a multiplet at d 7.59-8.14ppm corresponding to aromatic protons (Ar-H) and a singlet at d 11.98-12.03ppm corresponding to NH-proton; a singlet of three protons at d 3.88 ppm corresponding to N-CH3-protons. The composition of the atom present in the synthesized compounds was confirmed through element analysis.
The 13CNMR reveals the pic of the carbonyl group around 187.0ppm as the most deshielded.
Anti-microbial activity
In the recent times, many organisms are developing resistance to available antibiotics. Therefore, there is a need to prepare a novel agent that is resistant free in nature, acts specifically on microorganisms and causes the least harm to the host function. The synthesized chalcone derivatives possessed all the advantages as stated above due to the presence of pyridine and indole nucleus as part of the molecular structure. The synthesized compounds (2ac) had been screened for anti-microbial activity against Escherichia coli (ATCC 25922), Klebsiella pneumonia (ATCC 700603), Staphylococcus aureus (ATCC 25923), Proteus mirabilis, Salmonella typhi and Pseudomonas aeruginosa and Candida albicans using zone of inhibition (mm) and minimum inhibitory concentration (μg/mL) level. The amount of Compound 2d synthesized was not enough to be screen for antimicrobial activity and had just been used for structural analysis. We found that most of the synthesized compounds showed significant anti-microbial activity against different organisms and the results are demonstrated in Tables 1,2. Among the different synthesized compounds, compounds 2b showed significant antimicrobial activity against various organisms as compared to standard drugs like Norfloxacin and Nystatin. Particularly compound 2b had a zone of inhibition against S. typhi (20mm), P. mirabilis (19mm) and the lowest MIC value for S. typhi (31.25μg/mL) were recorded with compound 2c.
Compounds
S. aureus
E. coli
P. mirabilis
P. aeruginosa
S. typhi
K. pneumoniae
C. albicans
2a
10
9
18
8
9
8
20
2b
12
9.5
19
13
20
9
19
2c
9
9.5
17
12.7
17
7
21
Norfloxacin
19
15
22
17
16
12
-
Nystatin
-
-
-
-
-
-
23
Table 1: Diameter of inhibition of synthesized compounds against different micro-organisms (mm).
Compounds
S. aureus
E. coli
P. mirabilis
P. aeruginosa
S. typhi
K. pneumoniae
C. albicans
2a
250
500
125
125
1000
125
62.5
2b
125
62.5
125
250
62.5
125
125
2c
250
250
250
250
31.25
125
62.5
Norfloxacin
250
62.5
125
31.25
1000
31.25
-
Nystatin
-
-
-
-
-
-
15.625
Table 2: Minimum inhibitory concentration of synthesized compounds against different micro-organisms (μg/mL).
We noted that all the synthesized chalcones were more potent than the reference molecules on the test microbes, this is partly due to the fact that the a, β- unsaturated carbonyl group present on chalcones enhances the antimicrobial activity of chalcones, as, this structural feature was distinctive for all the synthesized products. This finding is in line with that of the literature [22-26]. When 2a’s hydrogen attached to the nitrogen atom is replaced by the methyl group (2b) growth inhibitory activity was markedly enhanced. The presence of the nitrogen atom at the para position in 2a and 2b seems to be playing a significant role in the expression of their activities.
The MBC/MIC ratio of a substance determines its ability to destroy or cause the death of the germ to which it is exposed. This ratio reveals that the tested compounds have bactericidal mode of action against gram positive and negative bacterial. The comparison of the zone of inhibition and minimum inhibitory of the synthesized compounds is shown in Figures 2,3.
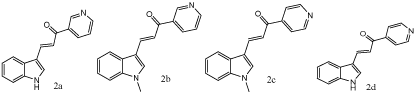
Figure 1: Chemical structures of synthesized compounds 2a-d.
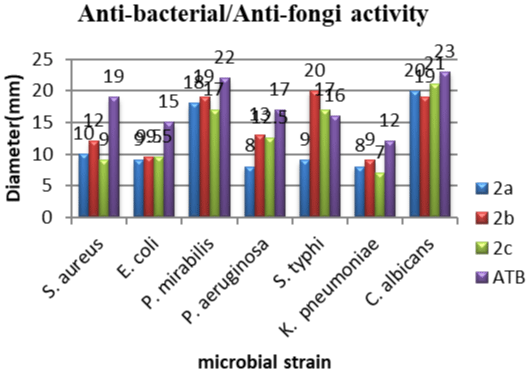
Figure 2: Anti-bacterial and anti-fungal zone of inhibition (mm) of synthesized
compounds against standard drug.
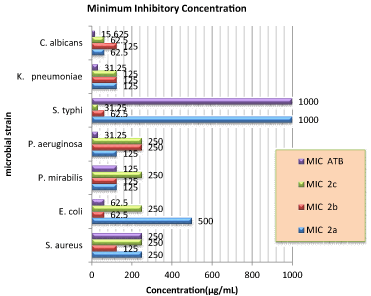
Figure 3: Anti-bacterial and anti-fungal minimum inhibitory concentration (μg/
mL) of synthesised compounds against standard drug.
Conclusion
This study stated a cheap and easy way of synthesized indole pyridine based chalcones from acetopyridine and substituted 1H-indole-3-carbaldehyde derivatives. The method employed was the Claisen-Schmidt condensation reaction in a reflux system with a weak base like piperidine. Compound 2b had shown good antimicrobial activities similar to those of a standard drug by the zone of inhibition and minimum inhibitory concentration. As shown in Figure 3, our results provided evidences that small structural changes may lead to dramatic difference in biological cell response.
References
- Abdula MA. Synthesis, characterization and antibacterial activity of (E)- chalcone derivatives. Eur J Chem. 2013; 4: 207-210.
- Kumar SK, Hager E, Pettit C, Gurulingappa H, Davidson NE, Khan SR. Design, synthesis, and evaluation of novel boronic-chalcone derivatives as antitumor agents. J Med Chem. 2003; 46: 2813-2815.
- Mokle SS, Sayyee MA, Chopde K. Studies on synthesis and antimicrobial activity of some new iodochalcones, flavones and flavonols. Int J Chem Sci. 2004; 2: 96-100.
- Ngameni B, Fokunang NC, Ambassa P, Azeh NN, Tembe-Fokunang E, Gonsu KH, et al. Synthesis and evaluation of antimicrobial properties of some chalcones. BJPR. 2016; 14: 1-16.
- Francesco E, Salvatore GM, Massimo C. Chemistry and pharmacology of oxyprenylated secondary plant metabolites. Phytochem. 2007; 68: 939-953.
- Liu M, Wilairat P, Go ML. Antimalarial alkoxylated and hydroxylated chalones: Structure-activity relationship analysis. J Med Chem. 2001; 44: 4443-4452.
- Hsieh HK, Lee TH, Wang JP, Wang JJ, Lin C. Synthesis and anti-inflammatory effect of chalcones and related compounds. J Pharm Res. 1998; 15: 39-46.
- Salman AS, Mahmoud NA, Abdel-Aziem A, Mohamed MA, Elsisi DM. Synthesis, Reactions and antimicrobial activity of some new-substituted indole Derivatives. Int J Org Chem. 2015; 5: 81-99.
- Miranda CL, Stevens JF, Ivanov V, McCall M, Frei B, Deinzer ML, et al. Antioxidant and prooxidant actions of prenylated and non prenylated chalcones and flavanones in vitro. J Agric Food Chem. 2000; 48: 3876-3884.
- Desti A, Majdalani M, Kontogiorgis CA, Hadjipavlou-Litina D, Kefalas P. Natural and synthetic 2’-hydroxy-chalcones and aurones: synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg Med Chem. 2009; 17: 8073-8085.
- Zhao LM, Jin HS, Sun LP, Piao HR, Quan ZS. Synthesis and evaluation of antiplatelet activity of trihydroxychalcone derivatives. Bioorg Med Chem Lett. 2005; 15: 5027-5029.
- Venkata RV, Rajendra PY, Ramarao N, Ramarao NV. Newer applications of 1,5-benzothiazepines and their cytotoxic and anticonvulsant activity. Der Pharm Lett. 2013; 5: 93-100.
- Murugan R, Anbazhagan S, Narayanan SS. Synthesis and in vivo antidiabetic activity of novel dispiropyrrolidines through [3+2] cycloaddition reactions with thiazolidinedione and rhodanine derivatives. Eur J Med Chem. 2009; 44: 3272-3279.
- Vanhoecke BW, Delporte F, Van Braeckel E, Heyerick A, Depypere HT, Nuytinck M, et al. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In vivo. 2005; 19: 103-107.
- Yadav BP, Ahmad I, Thakur M. Synthesis of some Novel indole derivatives as potential antibacterial, antifungal and antimalarial agents. IOSR Journal of Pharmacy. 2016; 6: 27-33.
- Altaf AA, Shahzad A, Gul Z, Rasool N, Badshah A, Lal B, Khan E. A Review on the medicinal importance of pyridine derivative. JDDMC. 2015; 1: 1-11.
- Pitucha M, Wos M, Miazga-Karsha M, Klimek K, Miroslaw B, Pachuta-Stec A, et al. Synthesis, antibacterial and antiproliferative potential of some new 1-pyridinecarbonyl-4-substituted thiosemicarbazide derivative. Med Chem Res. 2016; 25: 1666-1677.
- Trabbic CJ, Overmeyer JH, Alexander EM, Crissman EJ, Kvale HM, Smith MA, et al. Synthesis and biological Evaluation of Indolyl-pyridinyl-propenones having either methuosis or microtubiale disruption activity. J Med Chem. 2015; 58: 2489-2512.
- Gopi C, Dhanaraju MD, Sastry VG. Synthesis, characterization and biological evaluation of some novel 5-(benzotriazole 1-yl-methyl)-2-phenyl-1, 3, 4-oxadiazole azo compounds as an anti-microbial agents. Glob J Pharmacol. 2015; 9: 246-250.
- Gopi C, Sastry VG, Dhanaraju MD. Synthesis and spectroscopic characterisation of novel bioactive molecule of 3-(2-substituted)-1H-indol-3- yl)-1-(thiophen-2yl) prop-2-en-1-one chalcone derivatives as effective antioxidant and anti-microbial agents. JBAS. 2016; 5: 236-243.
- Alagarsamy V, Solomon VR, Krishnamoorthy G, Sulthana MT, Narendar B. Synthesis and anti-microbial activities of 1-(3 benzyl 4-oxo 3H quinazolin 2yl)- (-4-(substituted) thiosemicarbazide derivatives. J Serb ChemSoc. 2015; 80: 1471-1479.
- Padarthi PK, Sridhar S, Jagatheesh K, Namusivayam E. Synthesis and biological activity of imidazole derived Chalcones and its Pyrimidines. Int J Res Ayurveda Pharm. 2013; 4: 355-362.
- Falagas ME, Bliziotis IA. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007; 29: 630-636.
- Kuthiriya PJ, Purohit DM. Synthesis and antimicrobial activity of 2-{4’-[6’’-aryl) 2’’-amino-3’’, 4’’-dihydro-pyrimidine-4’’yl]-phenylamino}-6-[bis (2’’-chloroethyl) amino]-4-methoxy-1,3,5-triazine. J Chem Pharm Res. 2012; 4: 383-386.
- Manikandan D, Krishnaraj K, Nanjan MJ. Synthesis and biological evaluation of some substituted 4-piperidones. Int J Chem Res. 2012; 4: 532-538.
- Saify ZS, Faiyaz MV. Synthesis and anti-microbial sreening of some piperidine derivatives. Pakistan J Pharm Sci. 1998; 11: 15-21.