
Research Article
Austin J Anal Pharm Chem. 2019; 6(2): 1119.
In Vitro Quality Evaluation of Metformin Hydrochloride Tablets Marketed in Western and North Western Tigray, Ethiopia
Tesfay K1, Kahsay G2* and Dinda SC3
1Mekelle University, School of Pharmacy, Department of Pharmaceutics, Mekelle, Ethiopia
2Mekelle University, School of Pharmacy, Department of Pharmaceutical Analysis and Quality Assurance, Mekelle, Ethiopia
3College of Pharmacy, Teerthanker Mahaveer University, Delhi Road, Moradabad (UP), India
*Corresponding author: Kahsay G, Mekelle University, School of Pharmacy, Department of Pharmaceutical Analysis and Quality Assurance, Mekelle, Ethiopia
Received: June 11, 2019; Accepted: July 15, 2019; Published: July 22, 2019
Abstract
Objective: The study aims to evaluate the quality of Metformin hydrochloride (MET) tablets marketed in Western and North Western Tigray, Ethiopia.
Methods: Content determination was done based on the United States Pharmacopoeia (USP) method for MET using a reversed phase C18 (250mm x 4.6mm, 5µm) column. The mobile phase consisted of a mixture of acetonitrile and buffer (sodium heptane sulphonate and sodium chloride solution, pH = 3.85) in the ratio of 10:90 v/v. It was pumped at a flow rate of 1.0mL/min. UV detection wavelength was set at 233nm. Identification, weight uniformity, dissolution, hardness and moisture content tests were performed using methods stipulated in the USP. The dissimilarity factor (f1) and similarity factor (f2) were used to compare the drug release behavior of the dosages.
Results: Assay results showed that the average content of the drug products ranged from 98 to 101% and the dissolution rate was more than 70% in 45mins which were in agreement with the USP specification. Moreover, weight uniformity, hardness and moisture content of the products lie within the specification. Results of the f1 and f2 factors indicated that some generic brands showed deviations in drug release compared to the innovator brand.
Conclusion: In vitro quality evaluation of seven brands of metformin hydrochloride tablets marketed in Tigray with respect to identity, content, drug release, moisture content, hardness and uniformity of weight complied with the official specifications stipulated in the USP.
Keywords: Metformin; Quality; Evaluation; Analysis; Liquid Chromatography
Introduction
Metformin is an oral hypoglycemic agent which belongs to the class of drugs called biguanides. It is the first line drug for treatment and management of Type 2 diabetes mellitus particularly in overweight and obese people [1]. Metformin exerts its effect mainly by decreasing gluconeogenesis and by increasing peripheral utilization of glucose in skeletal muscles. The introduction of generic drug products from different manufacturing sources into the health care system of many developing countries was aimed to reduce the overall healthcare costs. However, this has been associated with various problems and among which the most perilous one is the distribution of counterfeit/falsified or substandard drug products [2]. Counterfeit medicines can be termed as branded or generic products which are deliberately and fraudulently mislabeled with respect to identity and source. This may include products with the correct ingredients or with the wrong ingredients, without active ingredients, with insufficient active ingredients or with fake packaging [3]. According to the WHO latest reports an estimated 10% medicines circulating in low-and middle income countries is either substandard or falsified with rates higher than 30% in sub-Saharan Africa [4,5]. What makes the fabrication, dissemination, and consumption of counterfeit/falsified pharmaceuticals very critical is that it grossly compromise nations’ healthcare system and which in turn leads to cause severe public health hazard with several overwhelming consequences [6].
In developing countries, counterfeit or substandard medicines are surprisingly fabricated by legal manufacturers, which do not meet the quality requirements set by national and/or international standards such as Good Manufacturing Practice (GMP) [7,8]. Further, lack of human and financial resources within the health sector as a whole limits the capacity of country’s drug regulatory agencies and results in a sub-optimally regulated environment in which substandard drug production can persist without detection [9,10].
It is therefore very mandatory to lay quality assurance mechanism to ensure the authenticity of pharmaceutical products. In vitro quality parameters such as drug content and dissolution tests are important quality indicators of solid oral dosage forms such as tablets. Dissolution profile studies are helpful to predict the bioequivalence of different brands, generics with themselves and/or with the innovator brand. Drug absorption process from solid dosage forms depends on the release of the drug substance from the drug product, the solubilization of the drug under physiological conditions and the permeability across the gastrointestinal tract [11,12]. Hence, dissolution tests and dissolution profile studies are crucial for the establishment of therapeutic equivalence of solid oral dosage forms [13,14]. In other words, in-vitro dissolution testing can be a valuable predictor of the in-vivo bioavailability and bioequivalence of oral solid dosage forms [15-17]. As diabetes mellitus is a serious public health problem, anti-diabetic drugs are among those highly prescribed medicines worldwide. Diabetes mellitus is the second most common non-communicable disease in Ethiopia [18]. To mitigate this problem different therapeutic options are being in use. Anti-diabetics in general and metformin hydrochloride tablets in particular are highly prescribed medicines in Ethiopia. There are different generic forms of metformin hydrochloride tablets available within the health delivery system globally as well as in Ethiopia after the expiration of patented or innovator brand called Glucophage. Literatures indicate that various brands of drugs with the same amount of active ingredient have shown differences in their therapeutic effect [19,20]. In addition to the one produced locally, Ethiopia has been importing different generic and innovator brands of the product from several countries. The aim of this study was therefore to assess the quality of different brands of metformin hydrochloride by evaluating critical quality parameters such as drug content, identity and rate of drug release. It is also important to compare the content, in-vitro dissolution and physicochemical properties of these generic brands with that of the innovator brand. This would eventually indicate whether generic substitution of this product illicit equivalent biological response as the originator brand. Moreover, quality evaluation of these drugs is crucial to ensure that patients get safe and efficacious medicines.
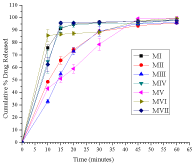
Figure 1: Dissolution profiles of six brands of metformin hydrochloride tablets
and the innovator product (MII) in phosphate buffer, pH 6.8. (In the figure
change cumulative to Mean).
Materials and Methods
Materials
Seven brands of metformin hydrochloride tablets coded as MI, MII (the innovator brand), MIII, MIV, MV, MVI and MVII were used in the present study. The generics were selected on the basis of being included in the Food, Medicine and Health care Administration and Control Authority of Ethiopia (FMHACA) 2018. Metformin hydrochloride tablets having labeled strength of 500mg were purchased from retail pharmacies found in West and North Western Tigray. All samples used for the study were within their shelf life during the time of evaluation.
Figure 2: Representative chromatogram of metformin hydrochloride tablets
of the sample MI.
Figure 3: Representative chromatogram of metformin hydrochloride
reference standard.
Instruments and equipment: Analysis was done using Agilent 1260 series HPLC system obtained from Agilent technologies (Waldbronn, Germany). The system is equipped with G1312B infinity binary pump, G4225A infinity high performance degasser, G1367E infinity high performance autosampler, G1316C infinity thermostat column compartment and G4212B Multi Wavelength Detector (MWD). Integration and analysis of the chromatographic peaks were carried out using the chemstation software version B.04. A Pharma test dissolution tester (Hainburg, Germany), UV-visible double beam spectrophotometer (Koyota, Japan), electronic balance (Sartorius, Germany), Hardness tester (Berlin, Germany), FTIR (Hainburg, Germany), pH meter (Hainburg, Germany), vacuum oven (Tuttlingen, Germany), Sonicator (Singen ,Germany) were generously provided by Addis Pharmaceuticals Factory.
Chemicals and reagents: Potassium phosphate mono basic was purchased from Riedel-de Haen (Baden-Wurttemberg, Germany), HPLC grade acetonitrile from Fisher Scientific Inter. Co (Loughborough, United Kingdom), Sodium hydroxide and ethanol from Fluka, Chemie, GmbH, (Stockholm, Sweden), sodium bicarbonate from Fluke Chemie, GmbH, (Zurich, Switzerland), sodium heptane sulphonate from Maple Biotech Pvt. Ltd. (Mumbai, India), and Sodium chloride from St. Louis, Missouri. (Missouri, USA), HPLC grade water and USP Metformin hydrochloride reference standard (RS) were generously given from Addis Pharmaceuticals Factory (Adigrat, Ethiopia).
Methods
Six generic brands of metformin hydrochloride tablets, each having labeled strength of 500 mg and the innovator brand were used in the study. Identification tests, weight uniformity test, water content, dissolution test and assay were performed as described in the United State Pharmacopeia [21].
Identification test: Identification test was performed by adding 20mL of ethanol to a 20mg powdered metformin hydrochloride tablets and the mixture was shaken and filtered. After the filtrate was evaporated to dryness, the residue was dried at 105ºC for 2h in a water bath to be utilized for identification test. The same procedure was followed for metformin reference standard. Then, the test sample was scanned in FTIR by KBR plate method. Finally, the spectra of the tablet (test sample) and the reference standard were compared.
Weight uniformity test: Twenty tablets from each brand were randomly selected and weighed collectively to obtain a mean weight. The tablets were then weighed individually and the percentage deviation of each tablet from the mean was then calculated.
Dissolution test: Dissolution profiles of seven brands of metformin hydrochloride tablets were evaluated directly after collection from the market. The test was performed by using dissolution tester type I (using paddle apparatus). To determine drug release profile, 900mL of phosphate buffer, at pH 6.8 was used as dissolution medium at 37±0.5°C. Samples were withdrawn at 10, 15, 20, 30, 45 and 60 minutes. The paddle was rotated at 100 Revolutions Per Minute (rpm). 10mL of samples were taken from each dissolution test vessel at each sampling time. Equivalent amount of a fresh 10mL dissolution medium was replaced immediately to maintain the vessel volume constant throughout the analysis. Each of the withdrawn samples were filtered with syringe filter 0.45μm. 1.5mL of the sample was diluted to 100mL of dissolution medium and the amount of dissolved metformin was determined by using UV-Visible spectrophotometer by taking absorbance at the wavelength of maximum absorbance at 233nm, Calibration curve for the metformin hydrochloride was done with similar conditions to be used as reference for drug content determination.
Hardness: Hardness tests were done for each brand by measuring the crushing strength of randomly selected ten tablets from each batch using hardness tester. The mean and standard deviations were calculated.
Moisture content: Moisture contents were determined following the method described in USP [21]. Ten randomly selected tablets from each batch were weighed and powdered. 1g of the powder was dried in vacuum oven at a pressure not exceeding 5mmHg at 100°C to constant weight. The percent mass lost after drying were calculated with respect to the mass before drying.
Assay of metformin hydrochloride tablets: High Performance Liquid Chromatography (HPLC) method was employed for the assay of different brands of metformin hydrochloride tablets.
Standard solution preparation: The standard solution of metformin was prepared by weighing accurately 50mg of metformin hydrochloride standard into a 100mL volumetric flask. About 50mL of diluent was added and sonicated to dissolve it completely. The prepared stock solution was filtered through a 0.45μm membrane filter. 5mL of this solution was diluted into 50mL flask with the diluent to obtain a standard solution with concentration of 50μg/mL (=100%).
Product
f1
f2
Remark
Generic A
19
38
Dissimilar
Innovator brand B
*Rb
*Rb
*Rb
Generic C
55
6
Similar
Generic D
41
16
Dissimilar
Generic E
50
12
Similar
Generic F
37
17
Dissimilar
Generic G
39
17
Dissimilar
Table 1: Calculated results of dissimilarity factor (f1) and similarity factor (f2) of metformin hydrochloride tablets using brand B as reference.
Brand Code No
Drug content
Hardness (N)
Moisture content
MI
100.55±0.30
171.30±1.12
1.78±0.10
MIII
98.12±0.120
209.66±3.20
0.39±0.12
MIV
98.35±0.100
194.28±4.30
1.40±0.12
MV
99.44±0.140
205.44±1.44
0.92±0.08
MVI
98.17±0.170
151.58±2.31
2.18±0.12
MVII
98.82±0.020
159.82±4.94
1.43±0.03
Table 2: Percentage of drug contents, hardness and moisture content (mean ± SD, n=3) of six different generics of metformin hydrochloride tablets.
Sample solution preparation: Twenty tablets were weighed separately and the average weight was determined. The tablets were finely powdered and a quantity of the powder equivalent to 50mg of metformin was transferred into 100mL volumetric flask. About 50mL of diluent was added and sonicated to dissolve it completely and made up to volume with the same solvent. The solution was filtered through a 0.45μm membrane filter. From this stock solution, 5mL was transferred into a 50mL volumetric flask and diluted upto the mark with diluent and mixed well to get a concentration of 50μg/mL of metformin hydrochloride.
About 10µL of standard preparation and sample solutions were separately injected into the chromatographic system and the peak areas for metformin were measured. Then, the percentage of metformin hydrochloride in the tablets was calculated.
Results and Discussion
Identification test of metformin hydrochloride tablets
Identification test is one of the most important tests that need to be carried out to unequivocally identify the API. The identity of the sample under test as well as the reference sample must be determined before further experimental work.
The reference standard was subjected to Infra-red (IR) scan as described in the method section. The reference IR spectrum of pure metformin hydrochloride is characterized by absorptions bands at the following wave numbers: 740, 935, 1075, 1063, 1580 and 1620cm-1 [22]. N-H wagging vibrations are assigned to absorption bands occurring at 740 and 935 cm-1; C-N stretch vibrations for 1063 and 1075 cm-1 and C=N stretch vibrations accounting for absorption bands at 1580 and 1620 cm-1 [23]. The IR spectrum obtained for the pure metformin hydrochloride showed absorption bands as in the aforementioned wave numbers.
Different generics of metformin hydrochloride tablets were also subjected to infra-red spectroscopy. The IR spectrum obtained for each metformin hydrochloride tablet had absorption bands at the following wave numbers 740, 935, 1075, 1063, 1620 and 1580 cm-1 similar to that of the reference spectrum. Hence, the identification test results indicated that all generics of metformin hydrochloride tablets used in this study contained metformin hydrochloride As Pharmaceutical Ingredient (API).
Weight uniformity test
Weight uniformity test was performed to check homogeneity among the units of the sampled batch. Tablets of different weights may differ in quality parameters, including the content of the active pharmaceutical ingredient. The mean weight of the tablets of the seven brands ranged from 559.12mg to 659.60mg. All brands showed acceptable weight uniformity as none of the tablets deviate by more than 5% from the respective mean weight of the brands as stated in the BP [24].
Dissolution test
Comparison of the therapeutic performance of two or more formulations containing the same active pharmaceutical substance is a critical means of evaluating the possibility of alternative use among the innovator and other similar formulated drug products [25,26]. Knowledge about the dissolution of a drug product can be used to ensure equivalence of the product in terms of drug release and post approval changes. Product with different formulation, different inactive ingredients and different formulation design may have different dissolution profile or release characteristics consequently may have different bioavailability. The USP stipulates that the amount metformin hydrochloride released within 45 min from the tablet should not be less than 70% of the stated amount.
As shown in Figure 1, all the generic products released about 70% of metformin hydrochloride within 45 min., hence, complying with the USP dissolution tolerance limit. In 15 mins, MV released 51.15%, MIII 54.80% and MII 65.71%. These products showed lower release properties compared with the drug release of MVII (95.73%), MIV (91.94%), MI (91.26%) and MII (86.74%).
The dissimilarity factor (f1) and similarity factor (f2) were used to compare the dissolution profiles as described elsewhere [27] using equations 1 and 2. Where in the equations, n is the number of testing time points; Rt is the average dissolution value of the reference product units at time t and Tt is the average dissolution value of the test product units at time t.
The guidance for equivalency is that the f1-values should be close to 0 (generally values less than 15) and the f2 value should be close to 100, with values greater than 50 ensuring equivalency. If the two dissolution profiles are exactly the same the f2 value will be 100. As the f2-value gets smaller there is a greater difference between the two profiles.
An f2 of 50 represents a 10% difference. Table 1 shows that only two generics (generic C and E) had f2 values more than 50. In other words, only generic C and E showed better similarity to the reference brand. Hence, these products can be considered to have similar drug release profile or bioequivalence with the reference brand B. The values (below 15) of dissimilarity(difference) factors for generics C and E also showed no major difference in terms of release of active pharmaceutical ingredient with respect to the reference drug. From this, it can be suggested that generics C and E could possibly be used interchangeably with the reference (brand B) since they had acceptable f1 and f2 values.
Assay of metformin hydrochloride tablets
Drug content determination helps to assure the presence of the required amount of active ingredient as claimed by the manufacturer. Significant variations could lead to ineffective therapeutic drug levels or overdosing that may lead to toxicity [28]. The results of dosage assays presented in Table 2 showed that the average content of metformin hydrochloride ranged from 98.12% to 100.55%. These assay values lie within the specified metformin hydrochloride percentage content stipulated in the USP (95%-105%). Representative chromatograms of the reference and a sample are depicted in Figures 2 and 3.
Hardness
Tablets require a certain amount of strength or hardness to withstand the mechanical shocks of handling and transportation yet soft enough to be able to disintegrate properly after swallowing. Since there is also a relationship between hardness and dissolution rate of the tablets, it is essential that the hardness of the tablets is within the acceptable range. The mean values of hardness of the different generics of metformin hydrochloride tablets are depicted in Table 2. The lowest mean tablet hardness (151.58N) was observed for MVI and the highest value (209.66N) was obtained for MIII. Conventional compressed tablets that have crushing strength greater than 40N are generally considered acceptable [29]. The examined tablets showed a crushing strength higher than 40N.
Moisture content
Moisture in pharmaceutical products, either as the residual water from processing or as a result of exposure to high humidity may affect the chemical or physical stability of moisture-sensitive products [30]. The brand with the least moisture content was brand MIII and highest was brand MVI which consisted 0.39%, and 2.18%, respectively. Results of the drug content are shown in Table 2.
Conclusion
The quality of seven brands of metformin hydrochloride tablets marketed in Western and North Western Tigray was evaluated with respect to their identity, content, drug release, moisture content, hardness and uniformity of weight. All brands of metformin hydrochloride tablets investigated complied with the official specifications stipulated in the USP and BP. Results of the dissimilarity factor (f1) and similarity factor (f2) from the dissolution profile studies indicated that only generic brands C and E showed similar drug release profile with the innovator. Even though, all the generic products evaluated released about 70% of metformin hydrochloride within 45 min as stipulated in the pharmacopoia, there exist variations in their release profiles. Variations in the drug release of the tablets could make difference in bioavailability and hence could not guarantee interchangeability of the generic brands. This quests the need for in vivo bioavailability studies of the different brands of metformin hydrochloride tablets.
Conflicts of Interest
The autors have declared no conflicts of interest with the publication of this manuscript.
Acknowledgement
The authors would like to thank Sheba University College for the financial support and thanks are also due to Addis Pharmaceutical Factory (APF) and College of Health Sciences, Mekelle University for using their laboratory facilities to conduct the experimental work.
References
- Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, et al. Clinical Review of anti-diabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol.
- Adegbolagun OA, Olalade OA, Osumah SE. Comparative evaluation of the biopharmaceutical and chemical equivalence of some commercially available brands of ciprofloxacin hydrochloride tablets. Trop J Pharm Res. 2007; 6: 737-745.
- World Health Organization, Counterfeit medical products. Sixty-third world health assembly A63/23 provisional agenda item. 2010.
- Kelesidis T, Falagas ME. Substandard/Counterfeit Antimicrobial Drugs. Clin. Microbiol. Rev. 2015; 28: 443-464.
- World Health Organization, A study on the public health and socioeconomic impact of substandard and falsified medical products. 2017.
- Feno? S, Wilson M. Africa’s Counterfeit Pharmaceutical Epidemic: The Road Ahead. 2009.
- Dorlo T, Ravinetto R, Beijnen J, Boelaert M. Global operation tackles internet trade in fake drugs. BMJ. 2012; 345: 23-27.
- World health organization WHO Global Surveillance and Monitoring System SSFFC Medical. 2016.
- Caudron JM, Ford N, Henkens M, Mace C, Kiddle-Monroe R, Pinel J. Substandard medicines in resource-poor settings: a problem that can no longer be ignored. Trop Med Int Health. 2008; 13: 1062-1072.
- Newton PN, Green MD, Ferna´ndez FM. Impact of poor-quality medicines in the developing world. Trends Pharmacol Sci. 2010; 31: 99-101.
- Ferraz HG, Carpentieri LN, Atanabe SP. Dissolution profile evaluation of solid pharmaceutical forms containing chloramphenicol marketed in Brazil. Braz Arch Biol Technol. 2007; 50: 57-65.
- Kassaye L, Genete G. Evaluation and comparison of in-vitro dissolution profiles for different brands of amoxicillin capsules. Afr Health Sci. 2013; 13: 369-375.
- Dunne S, Shannon B, Dunne C, Cullen W. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacology and Toxicology. 2013; 14: 2-19.
- Helmy SA and Bedaiwy. In-vitro dissolution similarity as a surrogate for in-vivo bioavailability and therapeutic equivalence. Dissolution technologies. 2016; 32-39.
- Basmenji S, Valizadeh H, Zakeri P. Comparative in vitro dissolution and in vivo bioequivalence of two diclofenac enteric coated formulations. ARZNAD. 2011; 61: 566-570.
- Sakore S, Chakraborty B. In Vitro-In Vivo Correlation (IVIVC): A Strategic Tool in Drug Development. J Bioequiv Availab. 2011.
- Masaad Ahmed MA, Shayoub MEA, Maghrabi IA, Masaad NMA. In Vitro-In Vivo Correlation Study of A newly Formulated Effervescent Ciprofloxacin Tablets With reference Tablets. Int J Curr Res Chem Pharm Sci. 2016; 3: 1-12.
- Wondemagegn AT, Bizuayehu HM, Abie DD, Ayalneh GM, Tiruye TY, Tessema MT. Undiagnosed Diabetes Mellitus and Related Factors in East Gojjam (NW Ethiopia) in 2016: A Community-Based Study. J Public Health Res. 2017; 6: 834.
- Fujii A, Furukori NY, Nakagami T, Niioka T, Saito M, Sato Y, et al. Comparative in vivo bioequivalence and in vitro dissolution of two valproic acid sustained-release formulations. Drug Des DevTher. 2008; 2: 139-144.
- Scheube E, Adamy L, Cardot JM. Mycophenolatemofetil: Use of a simple dissolution technique to assess generic formulation differences. Dissolution technologies. 2012; 19: 52-58.
- United States Pharmacopoeia: United States Pharmacopoeial Convention, Inc. Rockville, Marylan. 2013.
- Clarke’s Isolation and identification of drugs in pharmaceuticals, body fluids and post-martem material. 2nd edn. London (UK). The Pharmaceutical Press: 1986; 923.
- Gunasekaran S, Natarajan R, Renganayaki V, Natarajan S. Vibrational spectra and thermodynamic analysis of metformin. Indian Journal of pure Applied Physics. 2006; 44: 495-500.
- British Pharmacopeia: The Pharmaceutical Press, Her Majesty’s Stationery Office, London. 2013; 4.
- Esimone CO, Okoye OB, Onah CS, Omeje EO. In vitro bioequivalence study of nine brands of artesunate tablets marketed in Nigeria. J Vector Borne Dis. 2008; 45: 60-65.
- Hailu GS, Gutema GB, Hishe HZ, Ali S, Asfaw AA. Comparative in vitro bioequivalence evaluation of different brands of amoxicillin capsules armketed in Tigray, Ethiopia. IJPSN. 2013; 6: 1966-1971.
- Patel N, Chotai N, Patel J, Soni T, Desai J, Patel R. Comparison of in vitro dissolution profiles of oxcarbazepine-HP beta-CD tablet formulations with marketed oxcarbazepine tablets. Dissolution Technologies. 2008; 28-34.
- Akarawut W, Suvakontha T, Poompanich A. Pharmaceutical quality of nifedipine soft capsules commercially available in Thailand. J Health Sci. 2002; 11: 55-60.
- Allen LV, Popovich NG, Ansel HC. Ansel's pharmaceutical dosage forms and drug delivery systems, 8th Edition, Lippincott Williams and Wilkins, Philadelphia. 2004.
- Nanjwade BK, Ali MS, Manvi FV, Kanakal MM. Effect of tropical climatic conditions on the stability of cefaclor dry powder for suspensions. TJPR. 2010; 9: 73-79.