
Research Article
Austin J Anal Pharm Chem. 2019; 6(3): 1122.
Liquid Chromatography-Mass Spectrometry Studies on Molecular Structure of Melatonin after Co-60 Gamma Irradiation
1Department of Physics, Faculty of Basic Sciences, Imam Hussein Comprehensive University, Tehran, Iran
2Defense Chemical Research Lab (DCRL), Karaj, Iran
*Corresponding author: Babri M, Defense Chemical Research Lab (DCRL), P.O.BOX: 31585-1461, Karaj, Iran
Received: September 23, 2019; Accepted: October 30, 2019; Published: November 06, 2019
Abstract
Melatonin is known as a natural radioprotective agent. In this work, effects of gamma rays on melatonin molecular structure have been studied. Two sample groups, each has 6 specimens of melatonin in the form of a) powder and b) solution, were prepared. Selected samples were irradiated by the use of gamma cell radiation system under gamma-ray of Co-60 source, at doses from 20 to 2150 Gy. The content of irradiated sample vials compared with of the control samples using LC-MS and it revealed some structural changes in melatonin, although only in solution samples. Powder samples has not showed any significant changes after exposure. Observed changes in the chemical structure of melatonin in aqueous solutions, could be related to the production of free hydrogen and hydroxyl radicals; so both reduction and oxidation products were detected in sample solutions. Any increase in gamma ray doses would cause, proportional decrease in melatonin concentrations as well as increase in reaction products’ concentrations. The lack or low impact of gamma rays on solid melatonin are related to the low collision cross-section of melatonin for photons, as compared with the water molecules in aqueous solutions.
Keywords: Gamma Rays; Chemical Structure; Melatonin; Radioprotective Agent; LC-MS
- Abbreviations
MLT: Melatonin; LC-MS: Liquid Chromatography-Mass Spectrometry; ESI: Electrospray Ionization System; HPLC: High Performance Liquid Chromatography; AMU: Atomic Mass Unit
Introduction
Radioprotective agents
Radiation protection chemicals are compounds which are used to prevent, protect and treat living organisms against harmful damages of ionizing radiations. So, it is expected when they are applied before, during or after a human or animal exposure, the detrimental effects of ionizing radiation would be considerably reduced [1-4].
At some levels, organism’s immune system naturally is able to protect body against damages caused by radiations, and can detoxify and eliminate harmful effects of ionizing radiation. However, for high radiation doses, immune system cannot provide an acceptable level of protection [3].
Effectiveness of some compounds in protection against ionizing radiation was first discovered by Dale, Gray and Meredith in 1942, and their findings were published in 1948 [1]. In the early 1950s, the United States Atomic Energy Commission launched a program to make radioprotectors [1]. Despite the effectiveness of some chemicals in relatively short time, these compounds were almost toxic and caused nausea and vomiting at the protection doses [1]. Several years after acquiring more knowledge about the role of free radicals produced in various tissues of living organisms due to ionizing radiation and their effects on vital molecules, including DNA, a sort of mechanisms for radioprotection were explained [1]. Walter Reed Army Institute of Research started to perform a comprehensive program in order to synthesize some radioprotective agents during 1957-1986 [1]. An agent which was introduced through this program was a phosphorothioate compound known as amifostine, which is still one of the most effective radiation protection agent [1].
Natural radioprotective agents are selected by looking to their antioxidant and immune system stimulation activities [3]. The highest impact of these compounds should be the protection against biological damage and side effects of radiation toxicity [3,4]. Natural radioprotective agents have less protection potency than specially designed chemicals for such applications; however, their effects usually lasted more than synthesized chemicals. Also, their lower toxicity and side effects as well as the possibility of oral consumption, made them more suitable for radioprotection [2,3].
Today, the use of natural and specially designed radioprotective agents is necessary in radiotherapy and treatment of cancers, in order to protect healthy cells of patient’s body, as well as radiotherapists who may exposed during the operation [3].
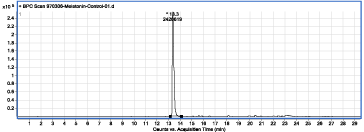
Figure 1: Chromatogram of the MLT control sample solution.
MLT, a radioprotective agent
As a natural radioprotective agent, MLT (N-acetyl-5-methoxytryptamine) is one of the hormones of pituitary gland which is synthesized in the pineal gland in human brain and quickly enters the bloodstream and through blood to other body fluids such as bile, cerebrospinal fluid, saliva, the follicular fluid of the ovary and semen [5]. Unlike amifostine, MLT could easily pass through the blood brain barrier [6]. This chemical was discovered by Lerner et al. in 1958 [5], and its radioprotective effectiveness was demonstrated in 1993 [7]. After reaction with hydroxyl and peroxyl radicals, MLT is oxidized to melatonyl, and through molecular rearrangements, cyclic 3-hydroxymelatonin is produced [5]. Also, MLT is able to increase the activity of certain antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, glutathione reductase and catalase at the molecular level, and suppresses the activity of the pro-oxidative enzymes [5]. In addition, Cui et al. suggested other interesting properties for this chemical, specifically, its anti-inflammatory, and anti-apoptotic as well as its anti-proliferative effects and apoptosis induction in cancer cells [8]. Jang et al. introduced MLT as a radiation sensitizer in cancer cells to induce an increase in apoptosis, and as a protector of normal cells by regulation of gene expression and, consequently, the reduction of apoptotic activity [9]. Lissoni et al. found that MLT is a natural angiogenesis inhibitor in cancer cells that controls the tumor tissues as radiation dose during radiotherapy is increased [10].
The present study aims to investigate effects of Co-60 gamma radiation on chemical structure of MLT in solid form and aqueous solutions. Based on our literature survey, no scientific reports have been presented about the effects of gamma radiation on chemical structure of MLT. Therefore, results of this study may help us in the use/non-use of drugs containing MLT, in a) contaminated areas due to the actuation by an active agent; and in b) radioactive disasters caused by losing, theft or missing the active sources, and spread of radioactive contamination, for instance into medical centers.
Materials and Methods
Chemical and reagents
Pure MLT was procured from Sigma-Aldrich. Ethanol, HPLC grade, were from Merck.
Two sample groups were prepared: a) powder samples; 4ml vials each containing 40mg MLT, and b) aqueous samples; 4ml vials containing 1.5ml 100mg/L MLT solution. One sample in each group was taken as control sample.
Preparation of test samples: To prepare a 100mg/L solution of MLT, 10mg of MLT powder was weighed in a small beaker and 500μL of absolute ethanol was added to it. Then, the solution was poured into a 10ml volumetric flask. The weighing container was washed with a small amount of absolute ethanol, which was then added to the volumetric flask. The volume of the solution in the volumetric flask was increased to 10ml with addition of absolute ethanol. This solution was considered as a stock solution with a concentration of 1000mg/L. Then, with dilution using distilled water, 10ml 100mg/L solution of MLT was prepared and poured into six 4ml vials, each containing 1.5 ml sample.
The aliquot samples were stored at -20°C.
Irradiation of samples: Irradiation of the samples was carried out by Gammacell (GC-220) irradiation system from Nordion, Ottawa, Canada with a dose rate of 1.61Gy/sec and activity of 6744 Ci. Five powder and five solution samples were irradiated with gamma rays at doses of 20, 50, 120, 500, and 2150 Gy in 9sec, 28sec, 1min:11sec, 5min:8sec, and 22min:12sec, respectively. The irradiated samples were kept at -20°C away from the light.
Instrumentation: To investigate possible changes in molecular structure of MLT in irradiated solid and solution samples, LC-MS was used. This system consists of an Agilent 1200 series LC which has equipped with a zorbax eclips XDB-C18 solvent saver plus HPLC column, 150mm length, 3mm i.d. and 3.5mm particle size, at 25°C. The eluent composition under elution programming was prepared using A) 15mM formic acid solution in water and B) 15mM formic acid in acetonitrile. The optimized elution program was 0-3min, 5% B, followed by gradually increment of B percent in eluent, from 5 to 100% until 15min. The flow rate of elution solvent, and sample injection volume were 0.3ml/min and 1ml, respectively. Chromatographic peak purity assessment was done using most intense ions in MS spectra under each peak.
The MS data were collected using Agilent 6410 Triple Quad MS with an electrospray ionization system. ESI voltage was at 4kV, and MS scan range was between 70 to 700 m/z. Each scan lasted for 500msec.
In these experiments, soluble samples were examined directly, but solid samples were dissolved in water and examined at a concentration of 278mg/L.
No.
Irradiation dose (Gy)
Relative peak area at specified retention time
total peak areas
I, 11.6min
II, 12.0min
III, 12.8min
MLT 13.3min
1a
0
0
0
0
100
100
2
20
0
0
0
100
100
3
50
0
0
0
100
100
4
120
0
0.2
0.3
99.4
99.9
5
500
0
1.5
1.2
97.3
100
6
2150
1.6
1.6
0.9
95.1
99.2
Table 1: Relative LC peak areas in the chromatograms of the irradiated MLT sample solutions.
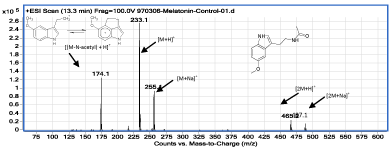
Figure 2: MLT ESI-MS spectrum.
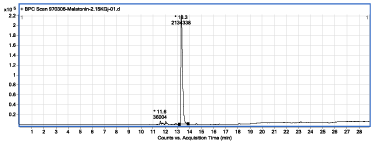
Figure 3: Chromatogram of the MLT sample solution, irradiated with gamma-ray at dose of 2150Gy.
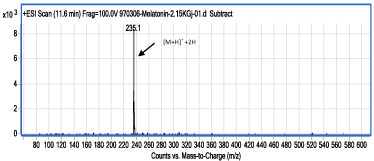
Figure 4: ESI-MS of spectrum of compound I (retention time: 11.6min); as a product of the irradiation of MLT solution by gamma rays at dose of 2150 Gy.
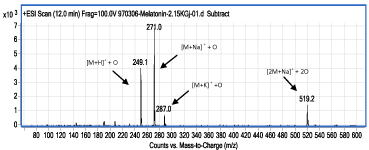
Figure 5: ESI-MS spectrum of compound II (retention time: 12.0min); as a product of the irradiation of MLT solution by gamma rays at dose of 2150 Gy.
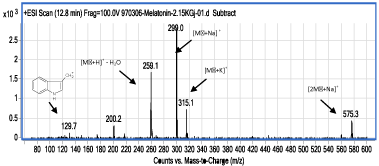
Figure 6: ESI-MS spectrum of compound III (retention time: 12.8min); at a product of the irradiation of MLT solution by gamma rays at dose of 2150 Gy.
Results and Discussion
LC-MS analysis of sample solutions
The MLT retention time as the major compound, under chromatographic condition is 13.3min (Figure 1). Based on structural information, the MS spectrum of MLT (Figure 2) was interpreted and identified ions were assigned.
Clearly, the content of an irradiated sample solution with gamma rays at dose of 2150Gy, in comparison with the control sample (Figure 1), was changed and some new peaks which are related to the formation of irradiation products are seen (Figure 3). Major irradiation products I, II and III are eluted from chromatographic column, at 11.6, 12.0 and 12.8 min, respectively. The focus of this report is on the identification of these compounds. Summary of analytical results obtained through the analysis of the other sample solutions are provided in Table 1. It is obvious that under even 2150 Gy gamma-ray irradiation, about 95% of MLT molecules, in aqueous solution, are intact.
LC-MS analysis of solid samples: The chromatograms and ESI mass spectra of irradiated and control solid samples showed no any difference, and are not presented in this paper.
Solution samples analyses: Considering the contents of Table 1, it can be concluded that by increasing the radiation dose, MLT concentration is relatively reduced and new compounds are formed which their concentration are gradually increased. A close look at chromatographic data (Table 1), shows that under exposure to gamma rays, a part of MLT is degraded into other compounds, mostly I, II and III. Due to the ESI-MS detection limit at the mentioned condition, some irradiation products can be seen in the solutions which were exposed at doses greater than 50Gy.
In order to identify product compounds using the ESI-MS spectra, without access to any MS library, not only probable ions’ fragmentation and formation processes, but also starting compounds and possible reasonable reactions should be taken into account.
The first possible reaction pathway is the radical degradation of water by gamma rays and creation of hydroxyl radical (•OH) and hydrogen atom. ESI-MS spectrum of the MLT irradiation product, I, which left the LC column at 11.6min (Figure 4), revealed that the molecular weight of this compound is only 2 unit more than MLT. Based on this fact, it was concluded that in compound I, one of the double bonds on MLT has converted into a single bond.
The compound whose ESI-MS spectrum provides enough evidence for the formation of oxidized form of MLT is the compound II. The mass spectrum of this compound and the results of the interpretation of this spectrum are provided (Figure 5). Compound II has an ESI-MS spectrum (Figure 5) in which most intense ions have found at 16 units higher m/z than the ions in the ESI-MS spectrum of MLT (Figure 2). Such data provide reasonable indication for the existence of the oxidized species of MLT in the solution. It is noteworthy that observation of adduct ions such as M+H [M: MLT molecule], M+Na, M+K, and dimer ions in LC-MS are usual [11], and those data together provide enough information to assign molecular weight for the interested molecule. Based on ESI-MS data, it is not possible to definitely assign the attachment point of oxygen atom on MLT; but Bonnefont-Rousselot, et al. stated that benzene like ring in MLT molecule is more ready for oxidation than the other parts of it [12]. Although it seems due to π-excessive ring, pyrrole ring in MLT is more attractive for •OH; another mechanism for MLT oxidation by •OH, has been proposed by Allegra, et al. in which another part of MLT is affected and a three cyclic compound is formed [13].
Mark-Trojanowicz et al., pointed out that due to water molecule radiolysis, the following species are formed, at the radiation point of action with corresponding radiation-chemical yield (G-value) which is expressed in mmol/J [14].
H2 O- ...→ •OH(0.28), eaq- (0.28), •H(0.062), H2 (0.047), H2O2 (0.073), H3O+ (0.28)
Upon formation, these and other secondary species are rapidly and homogeneously dispersed in the solution and may interact with other molecules, radical and ionic species, and are finally eliminated. The primary basic radicals, namely •H and •OH, quickly enter into acid-base equilibriums:
H•+HO-→eaq-+H2O
OH•+HO- O•-+H2O
Hydrogen radical (•H) and hydrated electron (e-aq) are in the acid-base equilibrium with pKa=9.1. In highly alkaline environments, •H atoms are converted to e-aq with the rate constant of 2.2x107 M-1sec-1. Since the process of electron or •H atom donation in water is slow, the lifetime of e-aq is relatively high [14].
Also, hydroxyl radical (•OH) is in an acid-base equilibrium with pKa = 11.8 with the O·- species. The rate constant of forward equilibrium reaction from hydroxyl to radical oxygen was reported to be 1.3x1010 M-1sec-1; and the rate constant for the reverse reaction equals to 7.9 x107 M-1sec-1 [14].
Simultaneous presence of reduced (I) and oxidized (II) forms of MLT as reaction products in the irradiated solution is an interesting point. With respect to the data shown in Table 1, concentration of those species in the solution are close together. This can be related to radical dissociation of water molecules due to irradiation. It has been showed that the oxidation/reduction properties of radicals resulting from the radiolysis of water are due to the following: a) The impact of •OH radical, which is a strong oxidant, and its oxidation potential varies due to solution pH from 11.9V for •OH/-OH to 12.72V for •OH, H+/H2O and; b) hydrated electron (e-aq) which is a powerful reducing agent in neutral and alkaline solutions with reduction potential of 22.87V [14]. In acidic media, •H is the major reducing agent with reduction potential of 22.31V. Such active species react with other molecules in water solution; and as an interesting finding, unlike other oxidizing or reducing processes, irradiation of water solution of a chemical might produce both the chemical’s oxidation and reduction products [14,15].
According to data collected from the ESI-MS spectrum of compound III (Figure 6), its molecular weight equals to 276 amu. The ions with the m/z 299 and 575 correspond to M′+Na and 2M′+Na, respectively. The ion which can be seen at m/z 259 is related to an ionic species which is obtained from the removal of a water molecule and addition of a proton to it, i.e. M′+H. The ion with m/z 315 seems to be potassium adduct of the molecule, M′+K. The molecular weight difference between this new species (M') and MLT is 44amu. Firstly, it should be noted that this compound has a hydroxyl group, due to the elimination of water in one of the ESI fragmentation process and formation of an ion with m/z 259. There is no such a group on MLT. So it might be a) a reaction product of reduced MLT, which may have a hydroxyl group, and or b) a consecutive oxidation product of MLT which have an acidic group which under ESI, loses a CO2 group. In analysis of these two possibilities it should be noted that the only reasonable position for reduction of MLT and formation of an alcoholic group, is the amidic C=O bond. Due to the presence of the ion which is seen at m/z 200, with 59amu difference between m/z of this ion and the ion at m/z 259 (Figure 6), and considering the ESI mass spectrum of MLT (Figure 2), it is found out that the amide group on compound III has remained intact. It seems that the ion which appeared at m/z 200 is formed after elimination of amide group from the structure of the ion at m/z 259. So the first possibility is rejected, or can be considered as the less effective process. To examine the second possibility, it must be kept in mind that CO2 origin should be in the gamma rays radiated aqueous solution of MLT. Since similar peak could not be seen in MLT control sample chromatogram (Figure 1), formation of compound III could not be attributed to the formic acid which is present in LC eluent composition. Bonnefont-Rousselot, et al., proposed a mechanism for oxidation of MLT in which formic acid is released [12]. According to Hart [18] formic acid in water solution under gamma radiation may produce active formyloxyl radical, HCOO•:
H•+HCOOHH2+HCOO•
OH•+HCOOHH2O+HCOO•
This radical, likely attacks to MLT to produce compound III with molecular weight of 276.
HCOO•+ (MLT-H)→(MLT-OOC)+H•
Note that in this reaction pathway, (MLT-H) and (MLT-OOC) are arbitrary symbols in order to represent single H atom and CO2 bonded to MLT.
Powder samples analyses: LC-MS analyses showed no any significant difference between the control sample chromatogram and chromatograms obtained from analysis of irradiated dry powder samples. This inactivity are attributed to the low cross section of MLT for gamma radiation.
The cross section is a convenient quantity to discuss the interactions of particles in matter [16]. In solution samples in which MLT molecules are dispersed in water, number of water molecules are much more than MLT molecules, and as a consequence, probability of the interaction of photons with atoms on water molecules is more than of MLT [16]. After aqueous solutions irradiation, and water radically dissociation, radical reactions are started. But in solid samples, despite the larger molecular dimension of MLT compared with water, the interaction between photons and atoms on MLT molecules are weaker than in solution. Mahfouz et al., reported a similar observation and a significant difference in the amount of irradiation products of solid iodophenol and its water-methanol solutions [17]. They reported in their study that the formation of products derived from radiolysis is mainly due to the formation of •H, •OH and e-aq. In fact, when photons collide to MLT atoms, an active species would not be obtained for the subsequent chemical reaction. Even, if reactive species are formed, reaction in a solid matrix may not proceed, due to lower space for motion and collision. In this study, analyses of solid MLT samples after irradiation revealed no any degradation product.
Conclusion
Based on findings of this research, it was concluded that A) with an increased dose of gamma radiation on MLT solutions, MLT structure are changed. After irradiation, MLT concentration is reduced and new compounds are formed and their concentrations gradually increased, in accordance with the received dose. B) MLT structural changes were seen in solutions which are irradiated by gamma rays with dose of 50Gy and greater. C) In this study, three different chemicals, reduced (I), oxidized (II), and CO2 adduct (III) forms of MLT were identified, using LC-MS. All product compounds were found in aqueous MLT solutions. D) After gamma rays exposure, chemical structure of dry powder MLT was not changed. It was mentioned that this finding is due to the lower cross section of MLT, as compared with water in aqueous samples.
References
- Foye WO. History of Radioprotector Development. Radioprotectors: Chemical, Biological, and Clinical Perspectives. In Radioprotectors, Chemical, Biological and Clinical Perspectives, Bump EA and Malaker K (Eds.) CRC Press. 1998; 15-24.
- Hosseinimahr SJ. Natural products with radiation protection. Mazandaran Journal of Medical Sciences. 2007; 17: 189-175.
- Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discovery Today. 2007; 12: 794-805.
- Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. The Oncologist. 2010; 15: 360-371.
- Reiter RJ, Tan DX, Herman TS, Thomas Jr CR. Melatonin as a radioprotective agent: a review. International Journal of Radiation Oncology Biology Physics. 2004; 59: 639-653.
- Leston J, Harthe C, Brun J, Mottolese C, Mertens P, Sindou M, et al. Melatonin is released in the third ventricle in humans. A study in movement disorders. Neuroscience letters. 2010; 469: 294-297.
- Meltz ML, Reiter RJ, Herman TS, Kumar S. Melatonin and protection from whole-body irradiation: survival studies in mice. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1999; 425: 21-27.
- Cui P, Luo Z, Zhang H, Su Y, Li A, Li H, et al. Effect and mechanism of melatonin's action on the proliferation of human umbilical vein endothelial cell. Journal of Pineal Research. 2006; 41: 358-362.
- Jang SS, Kim WD, Park WY. Melatonin exerts differential actions on X-ray radiation-induced apoptosis in normal mice splenocytes and Jurkat leukemia cells. Journal of Pineal Research. 2009; 47: 147-155.
- Lissoni P, Rovelli F, Malugani F, Bucovec R, Conti A, Maestroni GJ. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuroendocrinology Letters. 2001; 22: 45-48.
- Almeida EA, Klitzke CF, Martinez GR, Medeiros MH, Mascio PD. Synthesis of internal labeled standards of melatonin and its metabolite N1-acetyl-N2-formyl-5-methoxykynuramine for their quantification using an on-line liquid chromatography-electrospray tandem mass spectrometry system. Journal of Pineal Research. 2004; 36: 64-71.
- Bonnefont-Rousselot D, Collin F, Jore D, Gardès-Albert M. Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. Journal of Pineal Research. 2011; 50: 328-335.
- Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. The chemistry of melatonin's interaction with reactive species. Journal of Pineal Research. 2003; 34: 1-10.
- Trojanowicz M, Bobrowski K, Szreder T, Bojanowska-Czajka A. Gamma-ray, X-ray and Electron Beam Based Processes. In Advanced Oxidation Processes for Waste Water Treatment. Academic Press. 2018; 257-331.
- Allen AO. Mechanism of decomposition of water by ionizing radiations. Discussions of the Faraday Society. 1952; 12: 79-87.
- Tavernier S. Experimental techniques in nuclear and particle physics. Springer-Verlag Berlin Heidelberg. 2010; 23-45.
- Mahfouz RM, Siddiqui MR, Al-Wassil AI, Al-Resayes SI, Al-Otaibi AM. Chemical effects induced by γ-irradiation in solid and in aqueous methanol solutions of 4-iodophenol. Radiation Effects and Defects in Solids. 2005; 160: 173-180.
- Hart EJ. Gamma-Ray Induced Oxidation of Aqueous Formic Acid - Oxygen Solutions. Effect of Oxygen and Formic Acid Concentrations, Journal of American Chemical Society. 1954; 76: 4312-4315.