
Research Article
Austin J Anal Pharm Chem. 2020; 7(1): 1124.
Improvement of Dissolution Rate of Ketoprofen Using Solid Dispersions, Characterization and Dissolution Rate Comparison
Yusuf ATM1, Md. Shahidul I2* and Md. Jahedul I3
¹Department of Pharmacy, University of Science and Technology Chittagong, Bangladesh
²Assistant Professor, Department of Pharmacy, University of Science and Technology Chittagong, Bangladesh
³Human Resources Department, Healthcare Pharmaceutical Limited, Dhaka, Bangladesh
*Corresponding author: Md. Shahidul Islam, Assistant Professor, Department of Pharmacy, University of Science and Technology Chittagong, Bangladesh
Received: November 23, 2019; Accepted: January 07, 2020; Published: January 14, 2020
Abstract
Recently the challenging phases in the pharmaceutical industry are associated to strategies that improve the solubility of poorly soluble drugs. The manufacture of solid dispersions (SDs) is generally recognized as a method to improve the aqueous solubility, thereby increasing the oral bioavailability of drugs. In this study solid dispersion (SDs) of ketoprofen were prepared by solvent evaporation method using poloxamer 407 as carrier. Many hydrophilic carriers are used to increase solubility of poorly water drug. PEGs have the ability to solubilize some compounds and improve wettability. Solid Dispersion of drugs with PEG 6000 may be useful to increase stability, solubility, dissolution and bioavailability of poorly water-soluble drug. Solid dispersion technologies are used because it is particularly promising for improving the oral absorption and bioavailability of BCS Class II drugs. The purpose of this work was to increase the dissolution rate of ketoprofen by formation of solid dispersion with different water-soluble polymers. In this study, binary & ternary solid dispersion of ketoprofen were prepared with poloxomer 407, povidone K30, PEG 6000, HPMC at different ratios using the solvent evaporation method. In binary solid dispersion, drug-polymer ratio was 1:1, 1:3 & 1:5. In ternary solid dispersions drug was dispersed with mixture of PEG 4000, PEG 6000, lactose, poloxomer 407, povidone K30 and HPMC in different ratio where some time the amount of PEG 6000, lactose and HPMC was kept constant. The solid dispersions were investigated for drug loading & dissolution behavior. Solid dispersions showed a better dissolution compared to the pure drugs. Evaluation of the properties of the solid dispersion was also performed by using Fourier-transform infrared (FTIR) spectroscopy & Scanning Electron Microscopy (SEM). No interaction between drug & polymers was established by IR spectrum. So, solid dispersion technique may be an effective technique to enhance dissolution rate of ketoprofen.
Keywords: Ketoprofen; Solid dispersion; Poorly water-soluble; Poloxamer 407
Introduction
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on temperature and pressure [1]. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration where adding more solute does not increase the concentration of the solution [2]. Various approaches available to improve drug solubility as well as drug dissolution of poorly aqueous soluble drugs include micronization formation of inclusion complexes with cyclodextrins, formation of amorphous drugs and formation solid dispersions of drugs using various hydrophilic carriers. Among them, solid dispersion technique has attracted substantial interest as an efficient means of improving the dissolution rate as well as the bioavailability of a wide range of poorly aqueous soluble drugs [3]. The poor solubility and low dissolution rate of poorly watersoluble drugs in the aqueous gastro intestinal fluids often cause insufficient bioavailability. This may be achieved by incorporating the drug in a hydrophilic carrier material obtaining products called solid dispersions [4]. Depending on the properties of both, drug and carrier, and depending on their ratio, a solid solution or a solid suspension of the drug in the carrier material may be formed. The mechanisms involved in solubility and dissolution rate enhancement include transformation of unstable modifications into more stable ones or even into the amorphous state, reduction of particle size possibly to the molecular level as well as enhancement of wettability and solubility of the drug by the carrier material [5]. However, if a solid dispersion represents a thermodynamically unstable system, it is prone to convert into a more stable state [6]. Especially for substances according to the bio pharmaceutics Classification System, the bioavailability may be enhanced by increasing the solubility and dissolution rate of the drug in the gastrointestinal fluids [7,8].
Methods and Materials
Preparation of solid dispersion
The method of preparation of solid dispersions was based on the “solvent evaporation method”. The methods are described below:
In the solvent evaporation method of preparation, the carrier and the active ingredient are dissolved in the solvent- ethanol and the solvent is evaporated by magnetic stirrer. As the solvent is being removed, super saturation occurs followed by simultaneous precipitation of the constituents resulting in a solid residue. While preparing the solid dispersion of Ketoprofen, the drug solution was prepared by using drug, solvent (Acetone) and polymer (peg 6000, Poloxomer 407. At first Ketoprofen was weighed accurately in an analytical balance and taken into dry and clean glass vials. Then each polymer was taken in the vial. Then Acetone (0.5-3 ml) was added on each vial. Then drug and polymers are dissolved in Ethanol by sonication (5 minutes), vortexing (5 minutes) and then heating on magnetic stirrer until solvent evaporated and solid mass get. Drug, polymer, excipients and solvent combination was dried by using hair dryer until solid dispersion was formed. The solvent evaporated completely. Finally, the formulations were withdrawn from vials, crushed in mortar and pestle, and then passed through sieve (44 Mesh). Then the resulted samples were weighed and transferred in fresh vials with proper labeling. Formulations were kept in desiccators until the dissolution started.
Formulation of solid dispersion of ketoprofen by solvent evaporation method: Solvent evaporation method is common and mostly use in solid dispersion. Here various hydrophilic carriers are use (PEG 6000, Poloxomer 407. Drug and a single carrier are taken in a vial, and then properly dissolve by organic solvent acetone. At this stage, drug and carrier properly mixed with each other. Then solvent is evaporate by sonicator, water bath etc. It take some days to some weeks for form solid mass. After evaporate the solvent a solid substance is form, then grinding by mortar pestle, sieve mass size 40 and store in vial.
Formulation of ketoprofen solid dispersion by HPMC 15cps and changing the amount of povidone k30: Here drug mixed with double polymer. Fixed amount of HPMC 15cps mixed with drug and the amount of Povidone k30 is gradually chance from X1 to X5. After it that dissolve by solvent acetone. When homogenous mixture is obtain then evaporate the solvent by sonicator, water bath. For obtain solid mass keep in a desecrator. Then grinding, sieve and store in vial (Table 1).
Formulation
X1
X2
X3
X4
X5
ketoprofen
100mg
100mg
100mg
100mg
100mg
HPMC15 CPS
100mg
100mg
100mg
100mg
100mg
Povidone k30
500mg
400mg
300mg
200mg
100mg
Table 1: Formulation of Ketoprofen solid dispersion by HPMC 15cps and changing the amounts of Povidone k30.
In vitro dissolution study of Solid Dispersion
In vitro dissolution studies from the formulations were carried out for 1 hour in DI water. The solid dispersion was subjected to the paddle dissolution method using 900ml DI water. The dissolution test was performed at 75rpm and the temperature was set at 37°C ± 0.5°C. At different interval (5min, 15min, 30min, 40min, 50min & 60min) samples of 10ml were withdrawn, filtered and assayed by spectrophotometer at 258nm. After each sampling, equal volume (10ml) of fresh DI water with the same temperature was replaced. It was made clear that none of the ingredients used in the formulations interfered with the assay.
Preparation of phosphate buffer (7.4)
According to the various journal specifications, Phosphate buffer for Ketoprofen solid dispersion, here various PH ratios are use. The range is 5-7.4. But Ketoprofen is week acidic drug, for this reason PH range 6.8-7.4 is better. For prepare of phosphate buffer, 6.8g of potassium di-hydrogen phosphate (KH2PO4) dissolve in 1000ml distilled water. For maintain PH 7.4 Sodium Hydroxide solution is use.
Preparation of Standard Curve for Ketoprofen: To prepare a standard curve for Ketoprofen, 10mg of Ketoprofen was accurately weighed & dissolved in phosphate buffer in 100ml volumetric flask. Then take 10ml solution in 2nd volumetric flask. Add phosphate buffer up to 100ml. Then 1, 2, 3, 4, 5, 6, 7, 8, 9 & 10 ml of this solution is gradually taken in test tube & add gradually 9, 8, 7, 6, 5, 4, 3, 2, 1 & 0 ml phosphate buffer in those test tube for the purpose of serial dilution. This serial dilution was carried out to get different Ketoprofen concentration. These were then analyzed by UV spectrophotometer at 258nm and absorbance was noted. Then absorbance values were plotted against drug concentration and standard curve of Ketoprofen was produced.
Surface morphology study by scanning electron microscope (SEM)
Scanning electron microscopy was used to study the morphology and surface topology of the solid power particles. The solid particles from the optimized batch were mounted on the SEM sample stab (aluminium stabs) which were coated with a double sided sticking tape, sealed and finally coated with gold (200Å) under reduced pressure (.001 tor) for 15 minutes using ion sputtering device. The gold coated samples were scanned using scanning electron microscope (s-3400N, Hitachi) under different magnification and photomicrographs of suitable magnification were dried completely before examination.
Fourier transform infrared spectrophotometry (FTIR)
The IR spectrum of the pure drug and optimized solid dispersion formulation were obtained to prove the chemical integrity and compatibility of the drug in the solid dispersion. The sample (about 5mg) were powered and intimately mixed with 250mg of pure dry powdered potassium bromide (KBr) and the mixture was pressed into a disc using special mould and hydraulic press. The mixture were taken in a diffuse reflectance sampler and is spectra recorded by scanning in the wavelength region of 100 to 1000 cm in a FTIR spectrophotometer.
Result and Discussion
Dissolution rate studies of pure ketoprofen
Dissolution studies were performed using USP type ω apparatus. Samples of pure Ketoprofen, 50mg of ketoprofen were added to the dissolution medium 900ml of phosphate buffer saline pH 7.4 at a temp. 370C, the rpm was set at 75. In all experiments, samples was withdrawn at 0, 15, 30, 45, 60 min and replaced with an equal volume of the fresh medium to maintain a constant total volume. Samples were analyzed by U.V. spectrophotometer at 258nm.
Solvent evaporation method: Plot of X1, X2, X3, X4 and X5
Abbreviation:
• X-1= drug, HPMC 15cps and Povidone k30 (1:6)
• X-2= drug, HPMC 15cps and Povidone k30 (1:5)
• X-3= drug, HPMC 15cps and Povidone k30 (1:4)
• X-4=drug, HPMC 15cps and Povidone k30 (1:3)
• X-5=drug, HPMC 15cps and Povidone k30 (1:2)
Interpretation of release rate constants and R2 values for different release kinetics of X1, X2, X3, X4 and X5:
Table 2.
Formulation
Zero Order
First Order
Korsmeyer-peppas
K0
R2
K1
R2
n Value
X1
1.12
0.647
-0.015
0.909
0.19
X2
1.191
0.666
-0.016
0.897
0.263
X3
1.151
0.719
-0.012
0.89
0.308
X4
1.058
0.845
-0.009
0.945
0.398
X5
0.937
0.733
-0.008
0.883
0.237
Table 2: Release rate constants and R2 values for different release kinetics of five formulations of Ketoprofen Solid Dispersion.
The best fitted model and mechanism of drug release from X1, X2, X3, X4 and X5 batches of Ketoprofen Solid Dispersion:
Table 3.
Formulation No
Best fitted model
n Value
Release Mechanism
X1
First Order
0.19
Fickian transport
X2
First Order
0.263
Fickian transport
X3
First Order
0.308
Fickian transport
X4
First Order
0.398
Fickian transport
X5
First Order
0.237
Fickian transport
Table 3: The best fitted model and mechanism of drug release.
Pure Drug: In vitro dissolution study showed that percent drug dissolved after 5 minutes, 30 minutes and 60 minutes were 31.89%, 40.848%, 45.618% respectively.
X1: It was the batch of X1 (ketoprofen with HPMC 15cps and Povidone k30), Where Povidone k30 are in gradually changed. In vitro dissolution study shows the percent release of drug from matrix after 5 minutes, 30 minutes and 60 minutes were 56.08%, 77.49%, 89.79% respectively. In first order log cumulative % of drug remaining after 5 minutes, 30 minutes and 60 minutes were 1.644, 1.354, and 1.010 respectively
Best fitted model for this formulation is first Order (R2=0.909). The release mechanism of this formulation followed Fickian transport (n=0.190).
X2: It was the batch of X2 (Ketoprofen with HPMC 15cps and Povidone k30), Where Povidone k30 are in gradually changed. In vitro dissolution study shows the percent release of drug from matrix after 5 minutes, 30 minutes and 60 minutes were 45.96%, 80.48%, 90.77% respectively. In first order log cumulative % of drug, remaining after 5 minutes, 30 minutes and 60 minutes were 1.734, 1.292, and 0.967 respectively.
Best fitted model for this formulation is first Order (R2=0.897). The release mechanism of this formulation followed Fickian transport (n=0.263).
X3: It was the batch of X3 (Ketoprofen with HPMC 15cps and Povidone k30), Where Povidone k30 are in gradually changed. In vitro dissolution study shows the percent release of drug from matrix after 5 minutes, 30 minutes and 60 minutes were 38.74%, 74.74%, 84% respectively. In first order log cumulative % of drug remaining after 5 minutes, 30 minutes and 60 minutes were 1.788, 1.404, and 1.232 respectively.
Best fitted model for this formulation is first Order (R2=0.890). The release mechanism of this formulation followed Fickian transport (n=0.308).
X4: It was the batch of X4 (Ketoprofen with HPMC 15cps and Povidone k30), Where Povidone k30 are in gradually changed. In vitro dissolution study shows the percent release of drug from matrix after 5 minutes, 30 minutes and 60 minutes were 26.50%, 56.74%, 74.87% respectively. In first order log cumulative % of drug remaining after 5 minutes, 30 minutes and 60 minutes were 1.867, 1.637, and 1.401 respectively.
Best fitted model for this formulation is first Order (R2=0.945). The release mechanism of this formulation followed Fickian transport (n=0.398).
X5: It was the batch of X5 (Ketoprofen with HPMC 15cps and Povidone k30), Where Povidone k30 are in gradually changed. In vitro dissolution study shows the percent release of drug from matrix after 5 minutes, 30 minutes and 60 minutes were 39.64%, 56.38%, 71.53% respectively. In first order log cumulative % of drug remaining after 5 minutes, 30 minutes and 60 minutes were 1.782, 1.641, and 1.455 respectively.
Best fitted model for this formulation is first Order (R2=0.883). The release mechanism of this formulation followed Fickian transport (n=0.237).
Fourier transform infrared spectroscopy (FTIR) study
Fourier Transform Infrared Spectroscopy Study was conducted for following samples:
1. Pure drug Ketoprofen
2. Sample Ketoprofen, HPMC 15cps Povidone k30
Pure drug (Ketoprofen):
Figure 1.
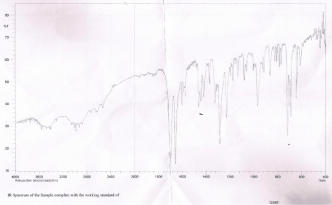
Figure 1: FTIR Spectra of pure Ketoprofen (Source: Incepta Pharmaceuticals
Limited).
Sample X2 (Ketoprofen, HPMC 15cps Povidone k30):
Figure 2.
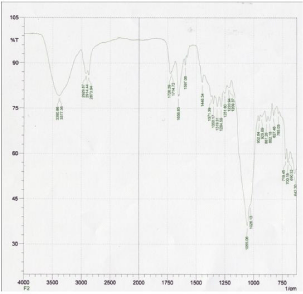
Figure 2: FTIR Spectra of X2 (Ketoprofen, HPMC 15cps Povidone k30).
Figure 1 represents the FTIR spectra of pore Ketoprofen. A lot of peaks are visible in these spectra. Ketoprofen exhibited general characteristic peaks at 2983-2930cm-1 (aromatic C-H stretch, carboxylic acid O-H stretch). The range of carboxylic acids O-H, stretch at 3400-2400cm-1.
Figure 2 represents the FTIR spectra of batch X2 (Ketoprofen, HPMC 15cps Povidone k30). Now if this spectra is compared with the spectra of Ketoprofen then we found that (aromatic C-H stretch, carboxylic acid O-H stretch) are present at 2929.87. (C-H stretch plus OH deformation), (aromatic C=C stretch) and (CHCH3 deformation), are present at 2873.94, 1597.06 and 1448.54 respectively. Another (carboxylic O-H out of plane deformation) and (C-H out of plane deformation for substituted aromatic) are presents at 1726.29 and 827.46 respectively.
Observation of particles morphology by scanning electron microscope (SEM)
The shape and surface morphology are important consideration for solid dispersion characterization. Scanning electron microscopic (Joel-LV-5600, USA, magnification of 250 x) photographs were obtained to determine crystal nature and morphology. SEM has been a process for ultrastructural analysis in the pharmaceutical industry. The characteristic properties of drug crystals like particle size and morphological surface can be known by the preparation method and chemical composition (Figure 3).
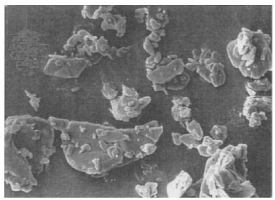
Figure 3: SEM of ketoprofen.
Effect of drug loading on shape and surface morphology
Ketoprofen, PEG6000, Poloxomer407 and lactose (Figure 4).
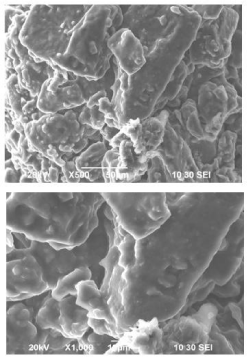
Figure 4: Effect of drug loading on shape and surface morphology of
Ketoprofen mix with PEG 6000 and Poloxomer 407.
Here scanning electron microscopy, (SEM) was used at different magnifications to determine the morphological differences of solid dispersions. SEM studies showed that the surface properties of drug with PEG 6000 and drug with PEG, poloxomer were lost in the process of preparing the solid dispersion system by melting and solidification causing the drug to be molecularly dispersed within the carrier matrix.
Conclusion
Many hydrophilic carriers are used to increase solubility of poorly water drug, Ketoprofen. Which has significance effects to increase solubility. Povidone is a Binder, Bioavailability Enhancer, and Taste- Masker. The active substance is embedded in a hydrophilic carrier. In solid solutions, the active substance is in an amorphous, molecular disperse form within the matrix. The role of HPMC as crystallization inhibitor and to prevent recrystallization of poorly soluble drug. Povidone is suitable for the manufacture of solid dispersions as it possesses hydrophilic properties, forms water-soluble complexes with many active substances, and is almost universally soluble. The present study has shown that it is possible to increase the dissolution rate of poorly water-soluble drug ketoprofen, by preparing a solid dispersion with water-soluble carriers, PEG 4000, PEG 6000, Poloxomer 407, Povidone k30 and HPMC. The solid dispersions show faster dissolution characteristics as compared to pure ketoprofen. Because the drug (Ketoprofen) can be rendered more soluble via solid dispersion system by the individual or synergistic, effects of particle size reduction, morphology alteration and improved particle and wetting properties by intimate association with hydrophilic carrier system. Since the absorption of ketoprofen from GI tract is primarily limited by the rate, at which they enter solution in the gastrointestinal lumen. Solid dispersion systems directly promote absorption of ketoprofen by accelerating their dissolution rate. The binary solid dispersion of ketoprofen in PEG 6000, Poloxomer 407 was effective in improving its dissolution properties. ketoprofen: PEG 6000 (1:5) which allowed achievement of 93% dissolved drug after only 30 minutes However, addition of a Surfactant (Poloxomer) when preparing the KP-PEG solid dispersions improved ketoprofen dissolution properties compared with the simple binary product. It can be concluded that with the carefully designed experimental technique, solubility of poorly water-soluble drugs can be improved by using Solid Dispersion technique. It would not be surprising that many types of solid dispersion of poorly water-soluble drugs based on these phenomena would enter into the market.
References
- Dahlberg C, Millqvist-Fureby A, Schuleit M. Surface comparison and contact angle relationship for differently prepared solid dispersion. Europ J Pharm bio-pharm. 2008; 70: 478-485.
- Singh S, Baghel RS, Yadav L. A review on solid dispersion. Int J Pharm Life Sci. 2011; 2: 1078-1095.
- Costantino HR, Firouzabadian L, Wu C, Carrasquillo KG, Griebenow K, Zale SE, et al. Protein spray freeze drying. Effect of formulation variables on particle size and stability. J Pharm Sci. 2002; 91: 388-395.
- Chiou WL, Riegelman S. Preparation and dissolution characteristics of several fast-release solid dispersions of griseofulvin. J Pharm Sci. 1969; 58: 1505–1510.
- Deepti, Dureja H, Madan AK. Solid dispersion adsorbates for enhancement of dissolution rates of drugs. PDA J Pharm Sci Tech. 2007; 61: 97-101.
- Bandarkar FS, Khattab IS. Lyophilized Gliclazide poloxamer solid dispersions for enhancement of in vitro dissolution and in vivo bioavailability. Int J Pharmacy Pharm Sci. 2011; 3: 122-127.
- Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971; 60: 1281-1302.
- Arunachalam A, Karthikeyan M, Konam K, Prasad PH, Sethuraman S, Ashutoshkumar A. A review on solid dispersion. Current Pharmaceutical Research. 2010; 1: 82-90.