
Research Article
Austin J Anal Pharm Chem. 2020; 7(1): 1125.
Design, Synthesis and Biological Evaluation of Novel Triterpenoid Derivatives Based on 20(S)-Protopanaxadiol as Potential Antibacterial Agents
Shao X1#, Zeng X1#, Zhou Y2, Zhang S1 and Zhou Z1*
¹Department of Pharmacy, Medical College, China Three Gorges University, Yichang, China
²Department of Quality Control, China Resources Sanjiu (Huangshi) Medical & Pharmaceutical Co., Ltd., Huangshi, China
#These authors contributed equally to this work
*Corresponding author: Zhiwen Zhou, Department of Pharmacy, Medical College, China Three Gorges University, Yichang, 443002, China
Received: November 25, 2019; Accepted: January 07, 2020; Published: January 14, 2020
Abstract
A new series of triterpenoid derivatives were synthesized based on 20(S)- protopanaxadiol (PPD) and evaluated for their antibacterial activity against several representative pathogens. Among which, compounds 5, 9, 11, 13 and 14 displayed good antibacterial activity against Gram-positive bacteria with MIC values of 2-16 μg/mL. Furthermore, additional testing against MRSA USA300 demonstrated that compounds 11, 13 and 14 also possess good antibacterial activity with MIC values of 2-8 μg/mL. The bactericidal effects revealed that compounds 13 and 14 displayed directly bactericidal activity against B. subtilis and MRSA USA300 with MBC values of 4-16 μg/mL. The subsequent synergistic activity assay of these derivatives was also carried out with results showing that compounds 13 and 14 could enhance the susceptibility of MRSA USA300 and B. subtilis 168 to kanamycin and chloramphenicol (FICI<0.5). Compounds 13 and 14 were then evaluated for their cytotoxicity and showed low toxicity with IC50 values about 30μg/mL against HeLa cells and about 95μg/mL against HEK-293 cells, respectively. The plausible structure-activity relationship was also concluded.
Keywords: Protopanaxadiol; Triterpenoid; Antibacterial activity; Synergistic effect; Cytotoxicity
Introduction
The discovery and development of antibiotics are among the most powerful and successful achievements of modern science and technology for the control of infectious diseases [1]. Many antibiotics and synthetic antibacterial agents such as nitrofuranes, cephalosporins, tetracyclines, macrolides and oxazolidinones are still in use today. However, as a result of the widespread and irrational application of antibiotics, multidrug resistance is now recognized as a global health problem. For instance, appearance of multidrug resistant Gram-positive bacteria, in particular, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) is still a serious menace [2-3]. Some of these strains are capable of surviving the effects of most, if not all, antibiotics currently in use [4]. These problems have highlighted the urgent need for designing and developing novel antibacterial candidates, which are distinct from those of traditional classes of antimicrobial agents.
Natural products have been the single most productive source of leads for the development of drugs, particularly as anti-cancer and antiinfective agents, such as taxol, berberine and allicin [5]. Furthermore, Antibacterial agents from natural sources generally possess complex architectural scaffolds and densely deployed functional groups, affording the maximal number of interactions with molecular targets, often leading to exquisite selectivity for pathogens versus the host [6]. The triterpenoids are the most representative group of phytochemicals, which are biosynthesized in plants through squalene cyclization. The diversity of triterpenes is highly associated with their broad range of pharmacological effects [7]. Active triterpenoids like oleanolic acid (OA), ursolic acid (UA) and petromyzanonamine disulfate (Figure 1), which are widely, occur in nature in free acid form or as an aglycone precursor for triterpenoid saponins have been reported to possess good antimicrobial activity [8,9]. Although few works have examined the mode of action of these triterpenes, studies conducted by Melzig showed that triterpenoids are likely to penetrate cell membranes by forming pore-like channels, leading a series of specific biological effects such as secretion processes, ion channel activation/ inhibition or change in the membrane structure [10]. However, the molecular mechanisms of actions between triterpenoids and the bacteria are not yet fully understood.
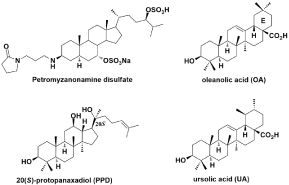
Figure 1: Structures of petromyzanonamine disulfate, OA, UA and PPD.
20(S)-protopanaxadiol (PPD) (Figure 1), a triterpene isolated from Fouquieria splendens Engelm, has been shown to have a variety of biological activities [11]. Previous reports indicated that PPD displayed moderate antibacterial activity with MIC values of 16 and 32 μg/mL against S. aureus RN4220 and B. subtilis 168, respectively. Furthermore, PPD could reduce the MIC of kanamycin against B. subtilis 168 from 0.25μg/mL to 0.0625μg/mL when combined with kanamycin, suggesting the strong synergistic effects between the two [12]. This discovery is highly potential for the development of new drugs in clinic to reduce the toxicity of antibiotics, specially the ones like kanamycin, which are currently second-line drugs as a result of their toxicity. We previously reported novel hydrophilic ocotilloltype triterpenoid derivatives with amino group at C-3 side chain that possessed good antibacterial activity [13]. Cationic triterpenoids have also been described in the literature that show a detergent like antibacterial effect which is generated by their amine-enriched structures [14,15]. Considering the above results and as part of our ongoing program in developing new active antimicrobials against resistant bacteria, herein, we described the synthesis and antibacterial activity of a novel series of hydrophilic triterpenoid derivatives bearing amino group at C-3 side chain based on PPD.
Results and Discussion
Synthetic chemistry
Applied methodology to produce compounds 1-6 is outlined in Figure 2. For the synthesis of the triterpenoid derivatives, the requisite starting material PPD was prepared from total ginsenosides by alkaline hydrolysis according to the literature [16]. As previously described [12], PPD was oxidized by excess of pyridinium chlorochromate (PCC) to provide dione 1, whereas 1M equivalent of PCC only oxidized 3-hydroxyl of PPD to obtain ketone 2. Regioselective protection of 3-hydroxyl of PPD obtained mono acetate 3. Oxidation or esterification of the 12-hydroxyl of 3, followed by deprotection of the acetic group produced target compounds 4 and 5. Additionally, interaction of PPD with excess of acetic anhydride generated diacetate, which was hydrolyzed in basic media to produce 6.
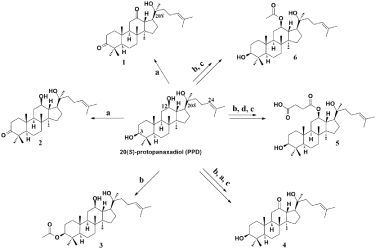
Figure 2: Synthesis of derivatives 1-6.
Reagents and conditions: (a) Anhydrous CH2Cl2, PCC, rt; (b) Anhydrous
pyridine, Ac2O, DMAP, rt; (c) CH3OH, KOH, rt; (d) Anhydrous CH2Cl2, succinic
anhydride, DMAP, rt.
The respective synthesis pathways of derivatives 9-14 are summarized in Figure 3. Treatment of ketone 2 with hydroxylamine hydrochloride in pyridine provided oxime 7, which was then reacted with epichlorohydrin yielded epoxide 8. Next, 8 was reacted with a series of amines of different structures to afford the required amino alcohols 9-14.
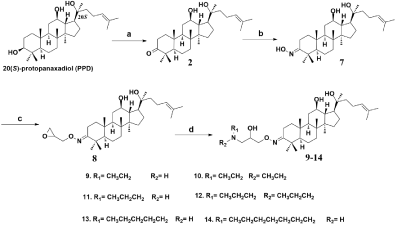
Figure 3: Synthesis of derivatives 9-14.
Reagents and conditions: (a) Anhydrous CH2Cl2, PCC, rt; (b) Anhydrous
pyridine, hydroxylamine hydrochloride, 700C; (c) Dimethyl formamide, NaH,
epichlorohydrin, 00C-rt; (d) Isopropyl alcohol, different amines, reflux, 5-10 h.
Antibacterial and bactericidal activity
The antibacterial activity of compounds 1-6 and 9-14 were evaluated against several representative Gram-positive (Staphylococcus aureus RN4220 and Bacillus subtilis 168) and Gram-positive (Escherichia coli DH5, Acinetobacter baumannii ATCC19606 and Pseudomonas aeruginosa PAO1) strains. Initial minimum inhibitory concentrations (MICs) screening results are presented in Table 1. The data showed that triterpenoid derivatives 5, 9, 11, 13 and 14 displayed good antibacterial activity with MIC values of 2-8 μg/mL against S. aures, and 2-16 μg/mL against B. subtilis, respectively. When the hydroxyl groups at C-3 and C-12 are both oxidized, the antibacterial activity of compound 1 significantly decreased from 16-32 μg/mL to 64-128 μg/mL against Gram positive bacteria. Compound 4, with hydroxyl group at C-12 oxidized, displayed enhanced antibacterial activity (16μg/mL) against S. aures compared to that of 2 (64μg/mL) which had a carbonyl group at C-3 position. For B. subtilis, both compounds 2 and 4 exhibited moderate antibacterial activity with MIC values of 32μg/mL. A comparative study of the activity of PPD, 1, 2 and 4 suggested that both C-3 and C-12 hydroxyl groups could influence the antibacterial activity of derivatives, and the effect of C-3 hydroxyl group is more obvious. However, when the hydroxyl group at C-3 or C-12 was acetylated, the antibacterial activity of compound 3 or 6 disappeared. Compared to the parent molecule PPD, compound 5 with aliphatic carboxylic acid group at C-12 side chain showed enhanced activity against S. aures and B. subtilis with MICs of 8μg/mL, respectively. Compounds 9-14, which were amino alcohols, showed good to moderate inhibitory activity with MICs of 2-32 μg/mL against Gram-positive bacteria. Among which, 13 and 14 were found to be the most active with MICs of 2μg/mL against S. aureus and 2, 4 μg/mL against B. subtilis, respectively. The structures of 9, 11, 13 and 14 are similar in all respects except for the length of C-3 side chain. A comparative study of 9, 11, 13 and 14 indicated that a specific chain length of C-3 can significantly influence the activity of the derivatives, and 8-10 carbon atoms at C-3 side chain is preferred. However, if there is a tertiary amine at C-3 side chain, compounds 10 and 12 displayed only moderate antibacterial activity with MICs of 32μg/mL against both S. aureus and B. subtilis. This result showed that the secondary amine group at C-3 side chain provided enhanced activity compared to that of the tertiary amine. Furthermore, only two derivatives, 11 and 13 showed moderate to mild activity against E. coli and P. aeruginosa with MICs of 32-64 μg/mL, which confirmed the capacity of these compounds to target Gram-negative bacteria, but further modification should be carried out.
Strain
S. aures
B. subtilis
E. coli
P. aeruginosa
A. baumannii
PPD
16
32
64
128
>128
1
64
128
128
>128
>128
2
64
32
>128
128
>128
3
128
128
128
>128
128
4
16
32
>128
>128
>128
5
8
8
>128
>128
128
6
128
128
>128
>128
>128
9
8
16
>128
>128
>128
10
32
32
>128
>128
>128
11
4
8
128
64
128
12
32
32
128
128
128
13
2
2
32
128
>128
14
2
4
>128
128
>128
KANa
1
0.25
1
8
1
aKAN: Kanamycin.
Table 1: In vitro antibacterial activity of the synthesized derivatives (MIC: μg/mL).
As shown in Table 2, the bioactive compounds against Grampositive bacteria were chosen for testing against a significant highly pathogenic methicillin-resistant strains S. aureus USA300 (MRSA USA300), which were also demonstrated to be quinolone-resistant. The results showed that compounds 11, 13 and 14 displayed good antibacterial activity against MRSA USA300 with MICs of 8, 2 and 2 μg/mL, respectively. However, compounds 5 and 9 could only exhibited moderate to mild activity against this pathogen with MIC values of 32-64 μg/mL.
Compounds
MRSA USA300
5
32
9
64
11
8
13
2
14
2
KANa
1
aKAN: Kanamycin.
Table 2: Antibacterial activity of triterpenoid derivatives against MRSA USA300 (MIC: μg/mL).
Compounds 5, 11, 13 and 14, which displayed promising antibacterial activity against Gram-positive bacteria, were then further investigated for their minimum bactericidal concentrations (MBC) and the data were listed in Table 3. Compounds 5, 13 and 14 displayed good bactericidal activity against B. subtilis with MBC values of 4-16 μg/mL, while compound 11 only kept moderate bactericidal activity with MBC value of 32μg/mL. For MRSA USA300, compounds 13 and 14 also exhibited directly bactericidal activity with MBC values of 4 and 16 μg/mL, respectively, while compounds 5 and 11 could only showed mild bactericidal activity with MBC value of 64-128 μg/mL. The bactericidal effects revealed the triterpenoid derivatives not only can affect bacterial cell viability, but also cause cell death, which could be as leads for further research.
Strain
B. subtilis
MRSA USA300
5
16
128
11
32
64
13
8
4
14
4
16
KANa
1
2
aKAN: Kanamycin.
Table 3: Bactericidal activity of 5, 11, 13 and 14 against B. subtilis and MRSA USA300 (MBC: μg/mL).
Synergistically antibacterial activity
Antibacterial agents targeting unrelated bacterial functions or processes may synergistically enhance their bioactivity when used in combination. Triterpenoid compounds are likely to display antibacterial activity by increasing the membrane permeability, which led to the leakage of intracellular constituents. Kanamycin is known as a bactericidal agent targeting the 30S of bacterial ribosome, while chloramphenicol controls the bacterial growth by inhibiting protein synthesis. As a result, the synergistic effects of compounds 13 and 14 were then investigated at their sub-MIC concentrations in combination with kanamycin and chloramphenicol against MRSA USA300 and B. subtilis. The effects were evaluated by calculating the Fractional Inhibitory Concentration Index (FICI) using fractional inhibitory concentration (FIC) [17]. As shown in Table 4, compounds 13 and 14 reduced the MICs of kanamycin against MRSA USA300 from 1μg/mL to 0.0078 and 0.032 μg/mL (FICI= 0.012, 0.048), respectively. For B. subtilis, strong Synergistic effects were also observed when combined 13 and 14 with kanamycin with FICI values of 0.009 and 0.017, respectively. Furthermore, when in combination of 13 and 14 with chloramphenicol, the MICs of chloramphenicol against MRSA USA300 were significantly reduced from 4μg/mL to 0.125 and 0.5 μg/mL, respectively. However, only an additive effect was observed when 13 and 14 were combined with chloramphenicol against B. subtilis (FICI= 0.50, 0.75). In contrast, when compounds 13 and 14 were combined with kanamycin, the MBC values of kanamycin significantly decreased from 4μg/mL to 1 and 2 μg/mL against MRSA USA300, respectively. For B. subtilis 168, potent bactericidal activity was also observed during the combination of 13 and 14 with kanamycin. Chloramphenicol alone was a bacteriostatic agent, but displayed promising bactericidal effect when combined with 13, with MBC value of 0.5μg/mL against B. subtilis 168. The above results suggested that these triterpenoid derivatives are suitable for combination with other antibiotics, specially the ones like kanamycin and chloramphenicol which are currently limited in use as a result of their toxicity.
Compound
MIC (μg/mL)
MBC (μg/mL)
FICI (FIC index)d
MRSA USA300
B. subtilis 168
MRSA USA300
B. subtilis 168
MRSA USA300
B. subtilis 168
KANa
1
0.25
4
1
-
-
CHLb
4
2
N/Ac
N/A
-
-
13+KAN
0.0078
0.002
1
0.25
0.012
0.009
14+KAN
0.032
0.0039
2
0.5
0.048
0.017
13+CHL
0.125
0.5
N/A
0.5
0.094
0.5
14+CHL
0.5
1
N/A
N/A
0.375
0.75
aKAN: Kanamycin.
bCHL: Chloramphenicol.
cN/A: Not applicable.
dFICI: According to the literature: FIC of drug A (FIC A)=MIC of drug A in combination/MIC of drug A alone; FIC of drug B (FIC B)=MIC of drug B in combination/MIC of drug B alone; hence FICI=FIC A+ FIC B. “Synergy” was defined when FICI was less than or equal to 0.5; while “additive” in which the FICI was greater than 0.5 and less than or equal to 1.0; whereas “indifferent” when the FICI was greater than 1.0 and less than or equal to 2.0; and “antagonistic” in cases which the FICI was greater than 2.0.
Table 4: Synergistic effect of antibiotics with compounds 13 and 14 against MRSA USA300 and B. subtilis 168.
Cytotoxicity assays
In order to determine the cytotoxicity of these synthesized derivatives, compounds 13 and 14, which displayed promising antibacterial activity, were chosen to test the cytotoxicity against human cervical (HeLa) and human epithelial kidney (HEK-293) cells by MTT assay. The results in Table 5 showed that both compounds 13 and 14 displayed low toxicity with IC50 values about 30μg/mL against HeLa cells and about 95μg/mL against HEK-293 cells, which suggested that these synthesized triterpenoid derivatives will not affect cell viability at their antibacterial MICs against Gram-positive bacteria.
Compound
IC50a, b (μg/mL)
HeLa
HEK-293
13
32.31±3.76
98.16±5.21
14
29.26±4.23
93.54±4.89
5-FUc
0.75±0.26
-
aIC50 is the concentrations required to inhibit 50% of cell growth.
bResults are expressed as the mean ± S.D. of three independent experiments.
c5-FU: 5-fluorouracil.
Table 5: Cytotoxic activity of compounds 13 and 14 against HeLa and HEK-293 cells.
Structure-activity relationship of the synthesized derivatives as antimicrobial agents
Based on the antibacterial activity of the synthesized triterpenoid derivatives, a plausible structure-activity relationship (SAR) could also be concluded. Hydrogen bond donors at C-3 and C-12 are required for activity against Gram-positive bacteria, while decreased activity was observed when the hydroxyl groups at C-3 and/or C-12 turned to ketone as hydrogen accepter. A non-hydrogen bond donor ester substitution at C-12 led to a loss of activity. However, the antibacterial activity increased significantly when the hydroxyl group at C-12 was replaced by acidic ester. For compounds containing an amino alcohol, a specific chain length of C-3 side chain can significantly influence the activity of the derivatives, and 8-10 carbon atoms at C-3 side chain is preferred. However, if there is a tertiary amine at C-3 side chain, compounds 10 and 12 only retained their moderate antibacterial activity with MICs of 32μg/mL against both S. aureus and B. subtilis, which was inferior to that of the compounds with secondary amine group at C-3 side chain.
Mechanism speculation
The modes of action of triterpenoid compounds were mainly related to cell membrane of bacteria. According to the literature [18], spontaneous formation of complexes between triterpenoids and cholesterol in membranes is followed by association of these complexes into ‘two-dimensional micellar-type structures’ within the membrane. The hydrophilic chains of the triterpenoids, which are thought to be centrally orientated in the micellar-like complex, lead to formation of an aqueous pore. These pores would cause an increase in membrane permeability enabling ions and macromolecules up to proteins to pass the membrane bilayer. Compounds 13 and 14 possessed good antibacterial activity, promising bactericidal effect and strong synergistic antibacterial activity which were suitable for the research of mode of action towards cell membrane system, and this part of the work is now in progress and will be reported in due course.
Experimental
General
Most chemicals and solvents were analytical grade and, when necessary, were purified and dried with standard methods. Melting points were determined on an XT-4 micro melting point apparatus and uncorrected.1H-NMR and 13C-NMR spectra were recorded respectively on Bruker AV-300 and Bruker AV-75 spectrometer using trimethylsilane (TMS) as an internal standard. The values of the chemical shifts are expressed in d values (ppm) and the coupling constants (J) in Hz. High-resolution mass spectra were recorded using an Agilent QTOF 6520.
General procedure for the preparation of 20(S)- Protopanaxadiol (PPD)
PPD were prepared from total ginsenosides by alkaline hydrolysis according to the literature [16].
(20S)-dammarane-24-ene-3ß, 12ß, 20-triol (PPD)
Mp. 196-1990C; ESI-MS m/z 461.5 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.15 (t, J=7.8Hz, 1H), 3.57 (t, J=5.0Hz, 1H), 3.19 (d, J=5.2Hz, 1H), 1.72 (s, 3H), 1.63 (s, 3H), 1.18 (s, 3H), 1.06 (s, 3H), 0.98 (s, 3H), 0.97 (s, 3H), 0.88 (s, 6H), 0.78 (s, 3H); 13C NMR (CDCl3, 75MHz) d 131.7, 125.0, 74.3, 71.7, 71.1, 55.8, 53.4, 51.6, 50.1, 47.7, 39.7, 39.0, 39.0, 37.1, 34.8, 34.5, 31.9, 31.2, 28.0, 27.3, 26.9, 26.4, 25.7, 22.3, 18.2, 17.7, 16.8, 16.1, 15.7, 15.3. HR-MS (ESI) m/z: calculated for C30H52.NaO3 [M+Na]+: 483.3814, found: 483.3818.
(20S)-dammarane-20-hydroxyl-24-ene-3,12-dione (1)
To a solution of PPD (50mg, 0.11mmol) in dry dichloromethane (8mL) was added pyridinium chlorochromate (65mg, 0.33mmol), the mixture was stirred at room temperature for 6h, then filtrated, concentrated in vacuo and the residue was purified over silica gel with petroleum ether-ethyl acetate (8:1) to give ketone 1 as a white solid (37mg, 75%). Mp. 168-1720C; ESI-MS m/z 457.4 [M+H]+; 1HNMR (CDCl3, 500MHz) d 5.20-5.30 (m, 1H), 2.94 (d, J=9.5Hz, 1H), 2.41- 2.63 (m, 3H), 2.20-2.30 (m, 2H), 1.39 (s, 3H), 1.24 (s, 6H), 1.21 (s, 3H), 1.09 (s, 3H), 1.04 (s, 3H), 0.90 (s, 3H), 0.87 (s, 3H); 13C NMR (CDCl3, 125MHz) d 216.7, 210.6, 131.6, 125.0, 71.0, 57.1, 55.7, 55.1, 52.5, 47.3, 40.3, 40.0, 39.2, 36.9, 36.4, 33.8, 33.5, 31.8, 29.4, 27.5, 26.9, 26.6, 26.1, 24.8, 24.0, 21.5, 19.6, 16.5, 15.7, 15.2. HR-MS (ESI) m/z: calculated for C30H48NaO3 [M+Na]+: 479.3501, found: 479.3509.
(20S)-dammarane-12ß, 20-dihydroxyl-24-ene-3-one (2)
To a solution of PPD (50mg, 0.11mmol) in dry dichloromethane (8mL) was added pyridinium chlorochromate (28mg, 0.13mmol), the mixture was stirred at room temperature for 4h, then filtrated, concentrated in vacuo and the residue was purified over silica gel with petroleum ether-ethyl acetate (4:1) to give ketone 2 as a white solid (39mg, 78%). Mp. 178-1810C; ESI-MS m/z 459.4 [M+H]+; 1HNMR (CDCl3, 500MHz) d 5.18 (t, J=7.6Hz, 1H), 3.55 (td, J=10.1Hz, 4.9Hz, 1H), 2.49-2.58 (m, 1H), 2.41 (dd, J=9.8Hz, 4.5Hz, 1H), 2.18-2.28 (m, 1H), 1.25 (s, 6H), 1.12 (s, 3H),1.09 (s, 3H),1.04 (s, 3H),1.00 (s, 3H), 0.98 (s, 3H), 0.92 (s, 3H); 13C NMR (CDCl3, 125MHz) d 217.5, 131.7, 125.3, 70.7, 70.1, 55.4, 52.0, 49.8, 49.5, 47.6, 47.2, 39.8, 39.6, 36.7, 34.1, 33.8, 32.2, 31.4, 31.3, 28.8, 27.9, 27.3, 26.7, 26.1, 24.8, 20.4, 19.7, 17.8, 16.2, 15.5. HR-MS (ESI) m/z: calculated for C30H50NaO3 [M+Na]+: 481.3658, found: 481.3660.
(20S)-dammarane-3ß-O-acetyl-24-ene-12ß, 20-diol (3)
To a solution of PPD (50mg, 0.11mmol) in dry pyridine (8mL) was added DMAP (24mg, 0.20mmol) and acetic anhydride (14mg, 0.14mmol), the mixture was stirred at room temperature for 4h, then extracted with ethyl acetate, and the organic layer was washed with 10% HCl, water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with petroleum ether-ethyl acetate (4:1) to give 3 as a white solid (40mg, 72%). Mp. 185-1880C; ESI-MS m/z 503.4 [M+H]+; 1HNMR (CDCl3, 300MHz) d 5.20 (t, J=7.8Hz, 1H), 3.58 (td, J=9.6Hz, 5.2Hz, 1H), 3.21 (d, J=5.6Hz, 1H), 2.21 (s, 3H), 2.12-2.18 (m, 1H), 1.27 (s, 6H), 1.15 (s, 3H), 1.10 (s, 3H), 1.06 (s, 3H),1.01 (s, 3H), 0.98 (s, 3H), 0.90 (s, 3H); 13C NMR (CDCl3, 75MHz) d 170.9,130.9, 126.1, 77.9, 72.5, 71.7, 56.2, 52.3, 50.7, 49.8, 46.7, 40.5, 39.9, 39.4, 38.9, 37.1, 35.2, 33.2, 29.5, 28.8, 28.1, 27.7, 27.0, 25.9, 24.6, 23.2, 22.1, 18.7, 17.7, 16.5, 15.7, 15.4. HR-MS (ESI) m/z: calculated for C32H55O4 [M+H]+: 503.4100, found: 503.4108.
(20S)-dammarane-12ß, 20-dihydroxyl-24-ene-3-one (4)
To a solution of PPD (50mg, 0.11mmol) in dry pyridine (8mL) was added DMAP (24mg, 0.20mmol) and acetic anhydride (14mg, 0.14mmol), the mixture was stirred at room temperature for 4h, then extracted with ethyl acetate, and the organic layer was washed with 10% HCl, water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with petroleum ether-ethyl acetate (4:1) to give 3ß-acetate as a white solid (40mg, 72%).
To a solution of 3ß-acetate (60mg, 0.12mmol) in dry dichloromethane (8mL) was added pyridinium chlorochromate (52mg, 0.24mmol), the mixture was stirred at room temperature for 8h, then filtrated, concentrated in vacuo and the residue was purified over silica gel with petroleum ether-ethyl acetate (8:1) to give 3ß-acetate-12-one as a white solid (46mg, 76%).
To a solution of 3ß-acetate-12-one (46mg, 0.09mmol) in methanol (10mL), Potassium hydroxide (11mg, 0.18mmol) was added. The reaction mixture was stirred at room temperature for 3h, then the methanol was evaporated and ethyl acetate was added. The organic layer was washed with water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with petroleum ether-ethyl acetate (4:1) to obtain 4 as a white solid (33mg, 81%). Mp. 172-1760C; ESI-MS m/z 459.4 [M+H]+; 1HNMR (CDCl3, 500MHz) d 5.25 (dd, J=7.5Hz, 3.8Hz, 1H), 3.20 (dd, J=10.9Hz, 4.8Hz, 1H), 2.91 (d, J=9.6Hz, 1H), 2.55 (td, J=9.3Hz, 4.2Hz, 1H), 2.15-2.20 (m, 2H), 1.21 (s, 3H), 1.20 (s, 3H), 1.15 (s, 3H), 1.12 (s, 3H), 0.98 (s, 3H), 0.93 (s, 3H), 0.88 (s, 3H), 0.82 (s, 3H); 13C NMR (CDCl3, 125MHz) d 211.4, 132.6, 125.0, 78.5, 71.2, 57.1, 55.8, 55.7, 54.3, 42.6, 40.9, 39.8, 38.9, 38.4, 37.7, 35.5, 34.7, 32.6, 29.7, 28.0, 27.6, 27.3, 26.6, 25.5, 24.7, 18.5, 16.7, 16.0, 15.7, 15.2. HR-MS (ESI) m/z: calculated for C30H50NaO3 [M+Na]+: 481.3658, found: 481.3660.
(20S)-dammarane-12ß-O-(3-carboxypropionyl)-24-ene-3ß, 20- diol (5)
To a solution of PPD (50mg, 0.11mmol) in dry pyridine (6mL) was added DMAP (24mg, 0.20mmol) and acetic anhydride (14mg, 0.14mmol), the mixture was stirred at room temperature for 4h, then extracted with ethyl acetate, and the organic layer was washed with 10% HCl, water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with petroleum ether-ethyl acetate (4:1) to give 3ß-acetate as a white solid (40mg, 72%).
To a solution of 3ß-acetate (60mg, 0.12mmol) in dry dichloromethane (10mL) was added DMAP (24mg, 0.20mmol) and succinic anhydride (24mg, 0.24mmol), the mixture was stirred at room temperature for 12h, then diluted with dichloromethane, the organic layer was washed with 10% HCl, water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with petroleum ether-ethyl acetate (1:1) to give 3ß-acetate-12ß-acidic ester as a white solid (49mg, 68%).
To a solution of 3ß-acetate-12ß-acidic ester (49mg, 0.08mmol) in methanol (10mL), Potassium hydroxide (5mg, 0.09mmol) was added. The reaction mixture was stirred at room temperature for 3h, then the methanol was evaporated and ethyl acetate was added. The organic layer was washed with water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with dichloromethane-methanol (30:1) to obtain 5 as a white solid (29mg, 65%). MP. 205-2090C; ESI-MS m/z 561.4 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.35 (dd, J=7.8 Hz, 2.3Hz, 1H), 3.86 (t, J=8.2Hz, 6.9Hz, 1H), 3.52 (dd, J=9.4Hz, 4.5Hz, 1H), 2.61-2.66 (m, 4H), 2.13- 2.21 (m, 1H), 1.89-2.05 (m, 2H), 1.27 (s, 3H), 1.24 (s, 6H), 1.08 (s, 3H), 0.97(s, 3H) , 0.91 (s, 3H), 0.88 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3, 75MHz) d 176.8, 172.1, 130.5, 126.3, 81.3, 70.6, 70.1, 56.3, 52.5, 50.9, 49.7, 48.2, 40.5, 39.3, 38.2, 37.5, 35.2, 33.0, 31.7, 31.6, 29.9, 29.5, 28.7, 28.3, 28.1, 27.7, 26.5, 25.3, 24.1, 18.5 (overlapping signal), 16.9, 16.7, 15.7. HR-MS (ESI) m/z: calculated for C34H57O6 [M+H]+: 561.4155, found: 561.4157.
(20S)-dammarane-12ß-O-acetyl-24-ene-3ß, 20-diol (6)
To a solution of PPD (50mg, 0.11mmol) in dry pyridine (8mL) was added DMAP (24mg, 0.20mmol) and acetic anhydride (42mg, 0.42mmol), the mixture was stirred at room temperature for 8h, then extracted with ethyl acetate, and the organic layer was washed with 10% HCl, water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with petroleum ether-ethyl acetate (10:1) to give 3ß, 12ß-diacetate as a white solid (48mg, 80%).
To a solution of 3ß, 12ß-diacetate (48mg, 0.09mmol) in methanol (10mL), Potassium hydroxide (6mg, 0.09mmol) was added. The reaction mixture was stirred at room temperature for 3h, then the methanol was evaporated and ethyl acetate was added. The organic layer was washed with water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with dichloromethane-methanol (4:1) to obtain 6 as a white solid (30mg, 66%). Mp. 187-1900C; ESI-MS m/z 503.4 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.21 (t, J=7.6Hz, 1H), 3.53 (td, J=10.1Hz, 4.9Hz, 1H), 3.18 (d, J=5.2Hz, 1H), 2.25 (s, 3H), 2.12-2.25 (m, 1H), 1.26 (s, 3H), 1.15 (s, 3H),1.11 (s, 3H), 1.01 (s, 3H), 0.99 (s, 3H), 0.97 (s, 3H), 0.89 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3, 75MHz) d 171.0, 132.5, 126.1, 78.9, 72.3, 70.7, 56.1, 52.8, 50.8, 50.1, 46.7, 40.1, 39.6, 39.2, 38.9, 37.1, 34.3, 31.2, 28.9, 28.2, 27.9, 27.2, 26.8, 25.9, 24.7, 23.5, 22.3, 18.9, 17.5, 16.5, 15.9, 15.4. HR-MS (ESI) m/z: calculated for C32H54NaO4 [M+Na]+: 525.3920, found: 525.3926.
General Procedure for the synthesis of 9-14
To a solution of 2 (100mg, 0.22mmol) in anhydrous pyridine (12mL), hydroxylamine hydrochloride (46mg, 0.66mmol) was added. The reaction mixture was stirred at 700C for 3h, then diluted by ethyl acetate. The organic solution was washed with 10% HCl, water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel to afford 7 (85mg, 82%).
To a solution of 7 (85mg, 0.18mmol) in anhydrous dimethyl formamide (12mL) was added sodium hydride (13mg, 0.54mmol) under 00C. After stirring at 00C for 0.5h, then epichlorohydrin (33mg, 0.36mmol) was added. The reaction mixture was stirred at room temperature for 5h, then diluted with ethyl acetate, the organic layer was washed with water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with petroleum ether-ethyl acetate (2:1) to give 8 as a white solid (71mg, 75%).
To a solution of 8 (60mg, 0.11mmol) in isopropyl alcohol (12mL) was added different amines (3 eq.). The mixture was refluxed for 5-10 h, then diluted with ethyl acetate, the organic layer was washed with water and brine, dried over anhydrous sodium sulfate, concentrated and purified over silica gel with dichloromethane-methanol (40:1- 10:1) to give the desired derivatives 9-14.
(20S)-dammarane-3-oxime-O-(2-hydroxyl-3-ethylamino propyl)- 24-ene-12ß, 20-diol (9)
White solid (49mg, 78%). Mp. 168-1720C; ESI-MS m/z 575.5 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.35 (m, 1H), 4.09 (dd, J=11.9Hz, 5.3Hz, 1H), 3.97 (td, J=11.4Hz, 5.8Hz, 1H), 3.86 (dd, J=10.5Hz, 5.2Hz, 1H), 3.47 (td, J=9.4Hz, 4.6Hz, 1H), 2.73-2.90 (m, 4H), 2.27-2.25 (m, 2H), 1.42 (s, 3H), 1.26 (s, 3H), 1.24 (s, 3H), 1.09 (s, 3H), 1.02 (s, 3H), 0.95 (s, 3H), 0.89 (s, 3H), 0.84 (s, 3H). 13CNMR (CDCl3, 75 MHz) d 165.1, 131.1, 127.4, 85.6, 81.5, 71.2, 70.4, 56.3, 52.3, 50.7, 49.6, 48.2, 41.6, 40.0, 38.9, 38.1, 37.3, 35.0, 32.8, 32.2, 31.5, 31.4, 30.4, 30.3, 29.9, 29.6, 28.8, 28.1, 27.7, 26.3, 23.9, 23.0, 17.3, 16.1, 15.8; HR-MS (ESI) m/z: calculated for C35H63N2O4 [M+H]+: 575.4788, found: 575.4787.
(20S)-dammarane-3-oxime-O-(2-hydroxyl-3-diethylamino propyl)-24-ene-12ß,20-diol (10)
White solid (50mg, 75%). Mp. 181-1850C; ESI-MS m/z 603.5 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.37 (m, 1H), 4.11 (dd, J=10.8Hz, 4.9Hz, 1H), 3.89 (td, J=11.0Hz, 4.9Hz, 1H), 3.80 (dd, J=9.3Hz, 4.2Hz, 1H), 3.42 (td, J=8.9Hz, 3.8Hz, 1H), 2.71-2.88 (m, 6H), 2.22-2.26 (m, 2H), 1.41 (s, 3H), 1.28 (s, 3H), 1.25 (s, 3H), 1.07 (s, 3H), 1.05 (s, 3H), 0.99 (s, 3H), 0.91 (s, 3H), 0.88 (s, 3H). 13C NMR (CDCl3, 75MHz) d 165.4, 131.3, 127.8, 84.9, 81.0, 71.5, 70.9, 56.4, 52.8, 50.9, 50.0, 48.6, 48.2, 41.2, 40.3, 38.8, 38.3, 37.6, 35.3, 32.6, 32.1, 31.6, 30.9, 30.5, 30.1, 29.9, 29.7, 28.6, 28.1, 27.8, 26.1, 23.8, 23.2, 17.4, 16.2, 15.8, 15.4; HR-MS (ESI) m/z: calculated for C37H67N2O4 [M+H]+: 603.5101, found: 603.5109.
(20S)-dammarane-3-oxime-O-(2-hydroxyl-3-propylamino propyl)-24-ene-12ß, 20-diol (11)
White solid (44mg, 68%). Mp. 170-1730C; ESI-MS m/z 589.5 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.31 (m, 1H), 4.01-4.03 (m, 1H), 3.98-3.99 (m, 1H), 3.78-3.83 (m, 1H), 3.44 (td, J=9.7Hz, 5.6Hz, 1H), 2.84-2.80 (m, 1H), 2.55-2.47 (m, 2H), 2.41-2.39 (m, 1H), 2.22- 2.20 (m, 2H), 1.36 (s, 3H), 1.25 (s, 3H), 1.16 (s, 3H), 1.05 (s, 3H), 1.03 (s, 3H), 0.97 (s, 3H), 0.89 (s, 3H), 0.83 (s, 3H). 13C NMR (CDCl3, 75MHz) d 164.6, 131.6, 127.4, 85.6, 81.5, 71.2, 70.4, 56.3, 52.3, 50.7, 49.6, 48.2, 41.6, 40.3, 38.9, 38.1, 37.4, 35.0, 32.8, 32.2, 31.6, 31.4, 30.5, 30.4, 29.9, 29.8, 29.6, 28.8, 28.2, 28.1, 27.8, 26.4, 25.2, 23.0, 16.2, 15.8; HR-MS (ESI) m/z: calculated for C36H65N2O4 [M+H]+: 589.4944, found: 589.4943.
(20S)-dammarane-3-oxime-O-(2-hydroxyl-3-dipropylamino propyl)-24-ene-12ß, 20-diol (12)
White solid (45mg, 75%). Mp. 180-1830C; ESI-MS m/z 631.5 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.30 (m, 1H), 4.10 (dd, J=10.5Hz, 4.3Hz, 1H), 3.88 (td, J=10.8Hz, 5.1Hz, 1H), 3.80 (dd, J=8.9Hz, 3.8Hz, 1H), 3.39 (td, J=9.1Hz, 4.0Hz, 1H), 2.68-2.78 (m, 6H), 2.19-2.24 (m, 2H), 1.40 (s, 3H), 1.29 (s, 3H), 1.24 (s, 3H), 1.08 (s, 3H), 1.03 (s, 3H), 1.01 (s, 3H), 0.95 (s, 3H), 0.87 (s, 3H). 13C NMR (CDCl3, 75MHz) d 164.8, 131.0, 127.5, 84.1, 81.1, 71.6, 71.1, 56.5, 52.7, 51.3, 50.6, 48.7, 48.4, 41.6, 40.8, 38.9, 38.5, 37.8, 35.6, 32.7, 32.4, 31.8, 31.1, 30.6, 30.1, 29.9, 29.7, 28.5, 28.0, 27.7, 26.6, 23.9, 23.4, 21.5, 21.1, 17.8, 16.5, 15.8, 15.5; HR-MS (ESI) m/z: calculated for C39H70N2O4 [M+H]+: 631.5414, found: 631.5412.
(20S)-dammarane-3-oxime-O-(2-hydroxyl-3-Pentamino propyl)- 24-ene-12ß, 20-diol (13)
White solid (47mg, 70%). Mp. 176-1780C; ESI-MS m/z 617.5 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.28 (m, 1H), 4.13-4.15 (m, 1H), 3.87 (dd, J=10.4Hz, 4.8Hz, 1H), 3.68 (td, J=10.1Hz, 5.5Hz, 1H), 3.31-3.26 (m, 1H), 3.21-3.14 (m, 2H), 2.89 ( d, J=15.6Hz, 2H), 2.27- 2.22 (m, 2H), 1.27 (s, 3H), 1.23 (s, 3H), 1.20 (s, 3H), 1.12 (s, 3H), 1.05 (s, 3H), 1.03 (s, 3H), 0.96 (s, 3H), 0.90 (s, 3H). 13C NMR (CDCl3, 75MHz) d 165.0, 129.1, 125.3, 85.6, 81.7, 71.2, 70.4, 56.3, 52.3, 50.7, 49.6, 48.2, 41.6, 40.3, 38.8, 38.2, 37.3, 35.0, 32.9, 32.2, 32.2, 31.4, 31.6, 31.4, 30.2, 30.0, 29.9, 29.6, 28.8, 28.2, 28.1, 27.8, 26.4, 25.2, 23.9,19.5, 16.2, 15.8; HR-MS (ESI) m/z: calculated for C38H69N2O4 [M+H]+: 617.5257, found: 617.5250.
(20S)-dammarane-3-oxime-O-(2-hydroxyl-3-heptamino propyl)- 24-ene-12ß, 20-diol (14)
White solid (50mg, 71%). Mp. 181-1830C; ESI-MS m/z 645.5 [M+H]+; 1H NMR (CDCl3, 300MHz) d 5.3 (m, 1H), 4.10-4.12 (m, 1H), 3.85-3.87 (m, 1H), 3.61 (m, 1H), 3.25-3.29 (m, 1H), 3.01-3.15 (m, 4H), 2.85 (d, J=15.1Hz, 2H), 2.19-2.23 (m, 2H), 1.27 (s, 3H), 1.24 (s, 3H), 1.19 (s, 3H), 1.13 (s, 3H), 1.05 (s, 3H), 1.01 (s, 3H), 0.98 (s, 3H), 0.92 (s, 3H). 13C NMR (CDCl3, 75MHz) d 164.8, 129.7, 125.8, 85.4, 81.2, 71.5, 70.8, 56.5, 52.8, 50.8, 49.9, 48.5, 41.5, 40.7, 39.1, 38.7, 37.8, 35.3, 32.9, 32.5, 32.2, 31.8, 31.6, 31.2, 30.9, 30.4, 29.8, 29.3, 28.8, 28.5, 28.1, 27.6, 26.8, 25.5, 24.1, 20.5, 19.5, 17.3, 16.4, 15.8; HR-MS (ESI) m/z: calculated for C40H73N2O4 [M+H]+: 645.5570, found: 645.5576.
Pharmacology
The antibacterial activity, synergistic antibacterial activity and cytotoxicity assays were performed as descried previously [12]. The minimum inhibitory concentrations (MICs) were determined against Gram-positive (Staphylococcus aureus RN4220, Bacillus subtilis 168 and MRSA USA300) and Gram-negative strains (Escherichia coli DH5, Acinetobacter baumannii ATCC19606 and Pseudomonas aeruginosa PAO1) using a standard LB medium dilution technique. The compounds possessing good antibacterial activity against Bacillus subtilis 168 and MRSA USA300 were then selected to determine their bactericidal activity against the same two pathogens, and kanamycin was used as a positive control. The cytotoxicity test of the synthesized compounds in vitro against human cervical (HeLa) and human epithelial kidney (HEK-293) cells were performed by MTT assay, and 5-fluorouracil was used as a positive control.
Conclusion
A new series of triterpenoid derivatives were synthesized based on PPD and evaluated for their antibacterial activity against several representative pathogens. Among which, compounds 5, 9, 11, 13 and 14 displayed good antibacterial activity against Gram-positive bacteria with MIC values of 2-16 μg/mL. Furthermore, additional testing against MRSA USA300 demonstrated that compounds 11, 13 and 14 also possess good antibacterial activity with MIC values of 2-8 μg/mL. The bactericidal effects revealed that compounds 13 and 14 displayed directly bactericidal activity against B. subtilis and MRSA USA300 with MBC values of 4-16 μg/mL. The subsequent synergistic activity assay of these derivatives was also carried out with results showing that compounds 13 and 14 could enhance the susceptibility of MRSA USA300 and B. subtilis 168 to kanamycin and chloramphenicol (FICI 0.5). Compounds 13 and 14 were then evaluated for their cytotoxicity and showed low toxicity with IC50 values about 30μg/mL against HeLa cells and about 95μg/mL against HEK-293 cells, respectively. These results suggested that triterpenoid derivatives based on PPD represent as potential leads for the development of antibacterial agents against antibiotic-resistant superbugs. Further investigations on mechanism of antibacterial action of these synthesized derivatives are currently under way and the results will be reported in due course.
Acknowledgments
The authors are grateful to Department of Pharmacology of China Three Gorges University for bioassay results.
Funding
This work was supported by China Three Gorges University [grant number 8000303].
References
- L Demain, RP Elander. The beta-lactam antibiotics: past, present, and future. Antonie Van Leeuwenhoek. 1999; 75: 5-19.
- JN Narendra Sharath Chandra, CT Sadashiva, CV Kavitha, KS Rangappa. Synthesis and in vitro antimicrobial studies of medicinally important novel N-alkyl and N-sulfonyl derivatives of 1-[bis(4-fluorophenyl)-methyl]piperazine. Bioogran. Med. Chem. 2006; 14: 6621-6627.
- PC Appelbaum, PA Hunter. The fluoroquinolone antibacterials: past, present and future perspectives. Int. J. Antimicrob. Ag. 2000; 16: 5-15.
- HW Boucher, GH Talbot, JS Bradley, JE Edwards, D Gilbert, LB Rice, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009; 48: 1-12.
- L. Harvey, Drug Discov. Today, 13, 894 (2008).
- S. B. Singh, J. F. Barrett, Biochem. Pharmaco. 71, 1006 (2006).
- Petronelli, G. Pannitteri, U. Testa, Anti-cancer drug. 20, 880 (2009).
- P. Y. Chung, P. Navaratnam, L. Y. Chung, Ann. Clin. Microb. Ant. 10, 25 (2011).
- H. S. Kim, S. N. Khan, J. R. Jadhav, J. W. Jeong, Bioorg. Med. Chem. Lett. 21, 3861 (2011).
- M. F. Melzig, G. Bader, R. Loose, Planta Med. 67, 43 (2001).
- J. B. Wan, Q. W. Zhang, W. C. Ye, Sep. Purif. Technol. 60, 198 (2008).
- Z. W. Zhou, C. Ma, H. Y. Zhang, Y. Bi, X. Chen, Eur. J. Med. Chem. 68, 444 (2013).
- Y. Bi, X. X. Liu, H.Y. Zhang, Molecules 22, 590 (2017).
- Y. Bi, C. Ma, H. Y. Zhang, Chem. Biol. Drug Des. 84, 489 (2014).
- B. Ding, Q. Guan, J. P. Walsh, J.S. Boswell, J. Med. Chem. 45, 663 (2002).
- G.J. Du, Q. Dai, S.Williams, Anti-cancer drug. 22, 35 (2011).
- B. C. L. Chan, M. Ip, H. Gong, S. L. Lui, R. H. See, C. Jolivalt, Phytomedicine, 20, 611 (2013).
- J. M. Augustin, V. Kuzina, S.B. Andersen, S. Bak, Phytochemistry, 72, 435 (2011).