
Research Article
Austin J Anal Pharm Chem. 2020; 7(1): 1127.
Development of Spectrophotometric Methods for the Determination of Nalbuphine Hydrochloride in Different Forms
Youssef S²*, Mostafa M² and Shereafy EE¹
¹Department of Chemistry, Menoufia University, Egypt
²Department of Forensic Chemistry lab, Ministry of Justice, Egypt
*Corresponding author: Sahar Youssef, Forensic Chemistry lab, Ministry of Justice, Forensic Medicine Authority, Egypt
Received: June 09, 2020; Accepted: October 17, 2020; Published: October 24, 2020
Abstract
Spectrophotometric methods for the quantitative determination of Nalbuphine Hydrochloride (N-HCl) in different forms which are raw samples, pharmaceutical preparation form and biological samples (spiked urine samples) were established. The proposed methods found to be simple, rapid, sensitive, precise and accurate. The determination proposed methods based on the reaction between nalbuphine hydrochloride in its different forms with coloring reagents forming colored ion associates, the ion associates exhibit absorption maxima in visible region which can be used in determination the concentration of Nalbuphine Hydrochloride (N-HCl). The used coloring agents are Bromophenol Blue (BPB) and Chlorophenol red (CPR) reagents. The optimum reaction conditions for quantitative analysis were investigated. In addition, the molar absorptivity and Sandell sensitivity were determined for the Nalbuphine Hydrochloride (N-HCl). The ion associates exhibit absorption maxima at 410 and 400 nm of (N-HCl) with BPB and CPR respectively. (N-HCl) could be determined up to 137.5ug/mL and 50ug/mL, using BPB and CPR respectively.
The correlation coefficient was ≥0.998 (n=6) with a relative standard deviation (RSD) ≤0.74 for five selected concentrations of the reagents. Therefore the concentration of N-HCl in its pharmaceutical formulations and spiked urine samples had been determined successfully.
Keywords: Naluphine Hydrochloride; Bromophenol Blue; Chlorophenol red
Introduction
Nalbuphine hydrochloride (5a,6a)-17-(Cyclobutylmethyl)-4,5- epoxymorphinan-3,6,14-triol hydrochloride, is narcotic analgesic drug which is a morphine- like drug with agonist activity at the k- opioid receptor and antagonist activity at the μ-opioid receptor. Nalbuphine is recommended for use in moderate to serve pain and its indications include pain after myocardial infraction.
There are several published research’s relating to methods for estimating Naluphine Hydrochloride (N-HCl) quantitatively in their dosage forms including spectrophotometric method [1-3], liquid chromatography mass spectrometry LC/MS [4], High Performance Liquid Chromatogrphy HPLC [5-7], gas chromatography [8] gas chromatography mass spectrometry GCMS [9], ion selective electrode [10].
In the present work the ion associate complex of nalbuphine hydrochloride (in raw samples, dosage form and in spiked urine samples) were studied spectrophtometrically. The proposed methods found to be selective, simple, rapid, sensitive, precise and accurate.
Experimental
Apparatus
Spectrophotometric measurements of N –HCl (Figure 1) with BPB and CPR reagents were measured using Agilent 8543 UV-Vis spectrophotometer equipped with quartz cell of 1 Cm optical path length with a resolution of 0.1 nm. The pH measurements of the prepared solutions were adjusted using HI 2211 benchtop pH meter HANNA. All spectrophotometric measurements were carried out at room temperature (25±2oC). Moreover, Millipore distillation apparatus model Direct Q3, France used for supplied deionized water.
chart :
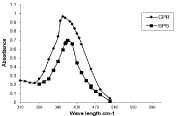
Figure 1: Absorption spectra of (N-HCl ) ion-associates with BPB and CPR.
Materials
The materials used in this study and their sources are tabulated in the following Table 1.
Material
Supplier
Naluphine hydrochloride
Amoun pharmaceutical industries Egypt
Naluphine ampoules (20mg/1mL ampoule)
Amoun pharmaceutical industries Egypt
methylene chloride) and sodium sulphate anhydrous ,
BDH
petroleum ether, diethyl ether, toluene, n-hexane, chloroform and cyclohexane
(Sigma-Aldrish
benzene)
Nasr chemical Egypt
Bromophenol blue
Leap chem co.Ltd
chlorophenol red)
(Kinbester co.Ltd
Table 1: The materials used in this study and their sources.
Parameter
N/Chlorophenol red (CPR
Bromophenol Blue (BPB)
λ max (nm)
400
410
Beer’s law up to (μgmL-1)
50.0
137.60
Molar absorpitivity (ε) (Lmol-1 cm-1)
0.84 x104
0.30 x104
Ringbom (μg mL-1)
3.17
18.0
Sandell sensitivity (μg cm-2)
0.047
0.13
Limit of detection(μgmL-1)
0.48
3.82
Limit of quantification (μgmL-1)
1.60
12.72
Color of ion- associate
Yellow
Yellow
Intercept
0.03
0.001
Slope
0.02
0.008
Correlation Coefficient
0.998
0.999
Table 2: Characteristics and analytical data of (N-HCl) ion-associates with CLPH and BPR.
Buffer solutions
Acetate buffer solutions were made of a mixture of 0.1M acetic acid and 0.1M sodium acetate trihydrate. Phosphate buffer solutions were made of a mixture of 0.1M disodium hydrogen phosphate, 0.1M HCl and 0.1M NaOH.
General procedure
5.0mL (2.0x10-3 M) of reagents (BPB and CPR) were added to different volumes of solution containing (2.0x10-3 M) of (N-HCl), and 2.0mL of buffer solution were added and the volume was completed to 10mL with distilled water. The formed ion–associates were extracted using the separating funnel with 10mL chloroform, the ion-associates were shaked for two minutes and allowed to separates into two phases. The organic layer was collected and dried with anhydrous sodium sulphate then completed to 10mL chloroform. The absorbance of the extract was measured at the recommended wavelength (λmax) .The blank solutions were prepared using the same method in absence of the examined drug. Linear curves were obtained by plotting absorbance versus concentration at respective λmax for each reagent. The calibration graphs were constructed and the concentrations of unknown samples were determined.
For the analysis of (N-HCl) in Naluphine ampoules (20mg/1 mL ampoule), three ampoules were diluted in 100 mL bidistilled water in a 100mL calibrated measuring flask., The drug content of these solutions was obtained by applying the general procedure to aliquot containing different volumes of drug solutions as described above.
For spiked human urine five milliliters of investigated drugs free urine taken in a separating funnel was spiked with different volumes of solution containing (2.0x10-3 M), N-HCl then 2.0mL of buffer solution were added and the volume was made up to 10mL with distilled water. (N-HCl) concentration was obtained by applying the general procedure to aliquot containing different volumes of solution drug as described above.
Composition of ion-associates
the molecular composition of (N-HCl) ion-associates with (BPB and CPR ) reagents were investigated , a series of solutions was prepared in which the reagent contents was kept constant, while that of the drug was regularly varied and the method was accomplished as previously mentioned in the general procedure. The absorbances of the resultant extracts were measured at the respective λmax of the ion-associates. The absorbance values were plotted against the molar ratio [drug]/[reagent] [11].
Job’s method of continuous variation method [12] was used; 2.0x10-3 M solution of investigated drugs was mixed with 2.0x10-3 M solution of each selected reagent. A series of solutions were prepared in which the total volume of drug and reagent was kept constant. The reagents were mixed with each drug in various proportions along with the chosen buffer solution, which then diluted in calibrated flask with the appropriate solvent following the above mentioned procedures.
Results
The optimum reaction conditions
selection of optimum wavelengths of maximum intensities (λmax), effect of pH, effect of extracting solvents, sequence of addition, effect of time, effect of temperature, stoichiometry of ion associates, and conditional stability constants, influence of foreign ions, effect of shaking time, number of extraction cycles and the amount of water-immiscible organic solvent were investigated to attain the optimum conditions to achieve maximum color development, for the quantitative determination of studied nalbuphine hydrochloride by using BPB and CPR reagents.
Selection of optimum wavelength (λmax)
The absorption spectra of N-H Cl ion associates with (BPB and CPR) were constructed as shown in Figure 1. The optimum wavelengths of maximum intensity (λmax) of (N-HCl) - BPB and CPR ion- associates were 410 and 400 nm for N-HCl ion associates with BPB and CPR respectively. The wavelengths maximum absorbencies (λmax) of the drug-coloring reagent ion-associates were recorded and tested against reagent blanks (prepared in the same manner without the addition of drug) to study the influence of each of the following variables on the formed ion associates between drugs and reagents.
Effect of pH
The effect of pH was studied to reach to the optimum pH values using acetate buffer solutions for each ion-associate. The optimum pH ranges for complete formation of the ion-associates which showed the highest absorbance values, at their respective λmax were, 4-6 with BPB, 3-5 with CPR as shown in Figure 2. From the results shown in Figure 2 we can observe that there are suitable ranges of stability of the absorbances of the drug- coloring reagent ion- associates which indicates that small change in pH will not effect on the efficiency of the formation of ion associates. So the proposed methods are suitable for determination of investigated drugs at different pH values.
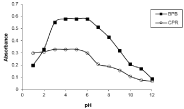
Figure 2: Effect of pH on (N -HCl) ion-associates with BPB and CPR.
Effect of extracting solvents
The type of solvent used affects both extraction efficiency and absorpitivity of the ion-associates, due to difference in the polarity Several water-immiscible organic solvents including cyclohexane, petroleum ether, n-hexane, diethyl ether, carbon tetrachloride, benzene, toluene, methylene chloride and chloroform to select the most convenient solvent of the highest absorbance. The aqueous to organic phase ratio of 1:1 was the most suitable for the ion-associate extraction. Complete extraction was attained by using one portion, of 10mL solvent on using the above reagents. It was found that chloroform is the most suitable solvent for the extraction of (N-HCl)- CPR-BPB ion-associates.
Suitable reagent concentration
The effect of reagent concentration was tested by using various amount of 2x10-3 M solution of CPR and BPB reagentS with 1 mL of 2x10-3 M of Naluphine hydrochloride. The results showed that 5 mL of of 2x10-3 M of BPB and CPR regent solutions were sufficient for the production of maximum and reproducible color intensity of the investigated ion-associates at their λmax. Excess of the reagents have no effect on the extracted fraction of the ion associates.
Suitable mixing sequence
Several different possible sequences of addition were checked to select the most suitable one for developing the most stable concerned ion-associates showed the highest absorptivity. The optimum sequence of mixing was found to be drug, reagent, buffer, and then solvent, which allow the highest color intensity and shortest time to obtain maximum absorbance.
Effect of time
The effect of time on the formation of the ion-associates was studied by measuring absorbance of the extracted ion-associates formed between its investigated drugs and the coloring reagents at increasing time intervals. The results showed that the ion-associates were formed almost instantaneously and the developed color remained stable for several hours which are 11 and 10 hrs for N-HCl ion associates with BPB and CPR respectively. After these intervals, a decrease in color intensity occurred. The effect of time on the stability of the ion-associates is represented graphically in Figure 3.
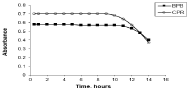
Figure 3: Effect of time on the stability of N-HCl ion-associates with BPB
and CPR.
The ranges of the stability of N-HCl-BPB and CPR ion- associates give the proposed methods the chance for undergoing all available studies without change in absorbance due to time factor.
Effect of temperature
With the above mentioned conditions the effect of temperature on the formation of the ion-associates of investigated drugs with coloring reagents was studied at temperature range 25-100oC. The results showed that the ion-associates were formed almost instantaneously in all cases at room temperature 25+5oC and remained constant up to different temperature ranges which are 50 and 55oC for N-HCl ion associates with BPB and CPR respectively. The effect of temperature on the stability of ion–associates is shown in Figure 4, the results show that the ion associates of N-HCl with BPB and CPR are stable in the temperature range (25oC-60oC) which are suitable to apply the proposed methods in different temperature ranges.

Figure 4: Effect of temperature on the stability of N-HCl ion-associates with
BPB and CPR.
The stoichiometry of the ion-associates
The stoichiometry of the ion-associates formed between N-HCl and BPB , CPR was investigated by the aid of the following recommended methods
The molar ratio method
For investigation the molecular composition of N-HCl ionassociates with BPB, CPR, serieses of solutions were prepared in which the reagents contents were kept constant, while that of the drugs regularly varied and the method was accomplished as previously mentioned in the general procedure. The absorbance of the resultant extracts was measured at the respective λmax of the ion-associates. The absorbance values were plotted against the molar ratio [drug]/[reagent](D/R) as shown in Figure 5 where straight lines were obtained intersecting at 2, that means the molar ratio was 2:1 (drug:reagent) and intersecting at 1, that means the molar ratio was 1:1 (drug:reagent).
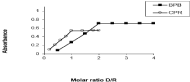
Figure 5: Molar ratio of of N-HCl ion-associates with BPB and CPR.
The continuous variation method
Series of solutions were prepared by mixing equimolar solutions of the drug and reagent in varying proportions while keeping the total molar concentration constant and the method was accomplished as previously mentioned in general procedure. The absorbance of the resultant extracts was measured at the respective λmax of the ionassociates. A plot of the absorbance versus the mole fraction of the drug is represented graphically in Figure 6. The curves exhibit a maximum at mole fraction 0.66 indicating that formation of 2:1 (drug: reagent). While maximum at mole fraction 0.5 indicating that formation of 1:1 (drug: reagent ) with the investigated drug. It was found that the stoichiometry of investigated drugs- coloring reagent ion-associates obtained from molar ratio results matched with those obtained from continuous variation results which confirm the obtained stoichiometry of the reactions by both methods.
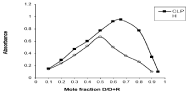
Figure 6: Continuous variation of of N-HCl ion-associates with BPB and CPR.
Conditional stability constant (kf ) of the ion-associates
The stability constants of the ion associates were evaluated. The formation of the ion- associates was rapid and the colored extracts were stable at least 7 hours for drug -reagent ion associates without any change in color intensity and the maximum absorbance at room temperature. The conditional stability constant (kf ) of the complex species of the ion- associates for the studied drugs with the coloring reagents were calculated from the continuous variation data using the following equation:
A/Am
kf = ___________
[1-(A/Am) ]n+2 CM (n)n
Where A and Am are the observed maximum absorbance and the absorbance value when all the drug is associated, respectively. CM is the mole concentration of the drug at the maximum absorbance and n is the stoichiometry with which dye ion associates with drug [13]. Calculated conditional stability constants were 4.35 and 4.13 for N-HCl ion associates with BPB and CPR respectively .In accordance with the formula the conditional stability constants reflected high stability of ion associates
Effect of interference
The effect of presence of foreign ions was studied by measuring the absorbance of the solutions containing 1mL of 2 X 10-3 M drug solution together with varying excess of different additives and excipients which may be present in the pharmaceutical preparations using the recommended methods of such reagents for N-HCl. No significant interference was observed from excipients commonly used such as glucose, lactose, starch, sucrose, magnesium stearate, methyl paraben and propyl paraben.
Shaking time
To study the effect of the shaking time for complete extraction of the ion-associates, the general procedure was applied after shaking the mixture for different time intervals (0.5-5.0 min). It was found that two min. is quite enough to complete the ion associate formation with highest absorptivity.
Number of extraction cycles
Number of extraction cycles was checked by applying the general procedure for single, double and triple extraction (each of two minutes) of the formed ion–associates. Complete extraction was attained by using one step of 10mL of solvent using selected reagent within the usable concentration range.
Aqueous phase: organic phase ratio
The amount of water-immiscible organic solvent was tested by using the general procedure in the sequence of addition of reagentdrug- solvent and different amount of organic solvent. The results revealed that a volume ratio of 1:1.5 (aqueous phase: organic phase) was enough for the quantitative extraction of ion-associates of the investigated drugs with the selected reagents.
Beer’s law verification and method validation
Linearity: The obedience of absorbance of the ion-associates of the N-HCl ion associates with BPB and CPR to Beer’s law is shown in Figure 7 The linear concentration ranges, the molar absorpitiveties (Є) were calculated and tabulated in Table 1 indicating high sensitivity of the reagents under investigation for the determination of N-HCl.
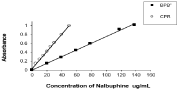
Figure 7: Standard curves of N-HCl ion-associates with BPB and CPR.
The regression equation (A=a+bc where A=absorbance, a=intercept, b=slope and c=concentration in μg mL-1), calculated from the calibration graph according to the Kaliedgraph program, were evaluated and recorded in Table 1. The intercepts of the lines were very small indicating that there is no systematic difference between the determined and expected concentrations within the investigated range using the present methods Figure 7. For more accurate results, ringbom concentration range was determined by plotting log [drug] in μgmL-1 against % transmittance from which the linear portion of the curve gives accurate range for the N-HCl Table 1.
Sensitivity
The detection limit (LOD) for the proposed method was calculated using the following equation
3S
LOD = ________
k
Where S is the standard deviation of replicate determination values under the same conditions as the sample analysis in the absence of the drug and k is sensitivity, namely the slope of the calibration graph. In accordance with the formula, the detection limits obtained for the absorbance were calculated and listed in Table 1. The limit of quantitation, LOQ, defined as:
10S
LOQ= ____________
K
According to this equation, the limits of quantification were calculated and listed in Table 1.
Precision and accuracy
In order to determine the accuracy and precision of the method, solutions containing four different concentrations of the studied drugs were prepared and analyzed in quintuplicate. Low values of Percentage relative standard deviation (RSD%) indicating good precision and reproducibility (repeatability) of the proposed methods Table 3. Precision was carried out by six determinations at four different concentrations using these spectrophotometric methods. Percentage relative error (Er%) as accuracy of the suggested methods was calculated using the following equation:
Intra-Day
Inter-Day
Method
Added μg mL-1
Recovery %a
Precision RSD%
Accuracy Er%
Confidence limitb
Recovery %
Precision RSD%
Accuracy Er%
Confidence limitb
25
99.37
0.72
-0.63
24.84±0.21
99.39
0.8
-0.61
24.85±0.23
(BPB)
50
99.65
0.4
-0.35
49.80±0.23
99.56
0.38
-0.44
49.78±0.21
75
99.73
0.27
-0.27
74.80±0.20
99.79
0.24
-0.21
74.84±0.20
100
99.61
0.18
-0.39
99.61±0.21
99.73
0.19
-0.27
99.73±0.21
10
98.35
0.17
-0.65
9.94±0.13
99.44
1
-0.56
9.94±0.12
CPR
20
99.45
0.13
-0.55
19.89±0.15
99.49
0.75
-0.51
19.90±0.17
30
98.25
0.4
-0.75
29.78±0.14
99.31
0.47
-0.69
29.79±0.16
40
99.38
0.35
-0.62
39.75±0.16
99.45
0.33
-0.55
9.78±0.153
n: Number of determination; RSD%: Percentage relative standard deviation; Er%: Percentage error. amean of five determination; bconfidence limit at 95% confidence level and five degrees of freedom.
Table 3: The intra-day and inter-day precision and accuracy of data obtained for determination of (N-HCl) by the proposed methods (n=6).
Found - added
Er % =[_________________] X100
Added
The inter–day and intra-day precision and accuracy results are shown in Table 3 indicate that the proposed methods are precise. The average percent recoveries were in range (98.25%-99.8%) indicating good accuracy of the proposed methods. These results showed that the proposed methods have good repeatability and reproducibility.
Robustness and ruggedness
For the evaluation of the method robustness, some parameters were interchanged; pH, reagent concentration, wavelength range, and shaking time. The capacity remains unaffected by small deliberate variations. Method ruggedness was expressed as RSD% of the same procedure applied by two analysts and using two different instruments on different days. The results showed no statistical difference between different analysts and instruments suggesting that the developed methods were robust and rugged Table 4.
Ion associate
Different analyst
Different instrument
X
±SD
RSD%
X
±SD
RSD%
N-CPR Pure N-HCl ( 30μg mL-1)
29.76
0.17
0.57
29.69
0.19
0.64
Naluphine 20mg per amp (30 μg mL-1)
30.07
0.11
0.37
30.02
0.14
0.47
Spiked urine sample (30μg mL-1)
29.58
0.13
0.43
29.51
0.12
0.41
N - BPB Pure N-HCl (30μg mL-1)
29.81
0.2
0.67
29.74
0.22
0.74
Naluphine 20mg per amp (30μg mL-1)
30
0.16
0.53
29.88
0.13
0.44
Spiked urine sample (30μg mL-1)
29.63
0.18
0.61
29.57
0.16
0.54
*: Theoretical value at 95% confidence level; n: Number of replicates.±
Table 4: The results of analysis of N-HCl obtained by two different analyst and instrument (n=6).
Analytical applications
The proposed methods were successfully applied to dosage forms and spiked urine samples of the investigated drugs. Six replicate determinations, using reported coloring reagents at different concentration ranges, were carried out for pure N-HCl and Naluphine ampoules (20mg/1 mL ampoule) and their spiked urine samples. The recovery values almost reach 100%, revealing a high accuracy of the results Table 5. The mean values obtained and the calculated standard deviations are compared with those obtained by the official methods [14,15], by applying the t- and F-tests [16,17] Table 6. Such comparison showed that there is no significance difference between proposed and the Official methods. This showed that high accuracy and precision of the proposed methods. So the present methods are accurate, precise, highly sensitive, rapid, and simple and their results are in good agreement with those of the official methods. Table 5 shows the results of the successful analytical applications of the suggested methods to the dosage forms of the investigated drugs and their spiked urine samples using the reported coloring reagents [18- 30].
Reagent
Pure solution
Naluphine 2 mg / amp
Spiked urine samples
Taken
μg mL-1
found μg
mL-1
Recovery%
Taken μg
mL-1
found μg
mL-1
Recovery%
Taken μg
mL-1
found
μg
mL-1
Recovery%
CPR
10
9.93
99.30
10
10.00
100.00
10
9.89
98.90
20
19.80
99.00
20
20.10
100.50
20
19.75
98.75
30
29.76
99.20
30
30.08
100.27
30
29.58
98.60
40
39.60
99.00
40
39.90
99.75
40
39.41
98.53
50
49.48
99.00
50
50.02
100.40
50
49.39
98.78
Mean recovery±RSD*
99.10±0.43
Mean recovery±RSD*
100.11±0.36
Mean recovery±RSD*
98.71±0.44
BPB
25
24.80
99.20
25
25.00
100.00
25
24.60
98.40
50
49.60
99.20
50
50.05
100.10
50
49.38
98.76
75
74.40
99.20
75
75.00
100.00
75
74.10
98.80
100
99.40
99.40
100
100.08
100.08
100
99.00
99.00
125
49.48
124.40
125
124.99
99.99
125
124.00
99.20
Mean recovery±RSD*
99.60±0.38
Mean recovery±RSD*
100.03±0.37
Mean recovery±RSD*
98.83±0.43
Table 5: Spectrophotometric determination of (N-HCl).
Parameters
Official method
N-BPB
N-CPR
Pure solution 40ug mL-1
X±SD
99. 40±0.16
99.25±0.15
99.38±0.17
n
6
6
6
t-value*
0.75
0.11
F-value
1.14
1.13
Naluphine 2 mg per amp 40ug mL-1
X±SD
99.82±0.1
99.80±0.15
100.05±0.11
n
6
6
6
t-value*
0.11
0.44
F-value
1.28
1.47
Spiked urine 40ug mL-1
X±SD
99.0±0.19
98.78 ±0.17
98.91±0.16
n
6
6
6
t-value*
0.87
0.4
F-value
1.28
1.14
*: Theoretical value at 95% confidence level; n: Number of replicates.
Table 6: Statistical treatment of data obtained for determination of (N-HCl) applying the proposed methods in comparison with the reference methods.
Conclusion
The proposed methods were made by using simple reagents, which most ordinary analytical laboratories can afford. The methods are sufficiently sensitive to permit determination of the investigated drugs at given optimum conditions in pure solutions, pharmaceutical preparations and spiked urine samples.
Unlike gas chromatography and high performance liquid chromatography, Spectrophotometry is relatively simple to handle and affordable.
The proposed methods are simple, precise, accurate, robust, rugged and low coast. Therefore, the proposed methods should be useful for routine quality control purposes and pharmaceutical industries.
References
- Parfitt K. “Martindale, the Extra pharmacopoeia”. The Pharmaceutical Press. London. 32nd edition. 1999.
- Mona E, El Sharkasy, Walash M, Belal F, Belal F. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2020; 2285: 117841.
- Belal F, Ibrahim F, Sheribah ZA, Alaa H. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2018; 1985: 51-60.
- Klinzig F, Vinner E, Brassart C, Houdain E, Humbert L, Lhermitte M. J Anal Toxicol. 2007; 31: 62-67.
- de Cazanove F, Kinowski J, Audran M, Rochette A, Bressolle F. J Chromatogr. 1997; 690: 203-210.
- Nicolle E, Veitl S, Guimier C, Bessard G. J Chromatogr. 1997; 690: 89-97.
- Shung-Tai Ho, Jhi-Joung Wang, Oliver Yoa-Pu Hu, Pei-Shan Chiang, Shih- Chun Lee. J Chromatogr. 1996; 678: 289-296.
- Yoo YC, Chung HS, Kim IS, Jin WT, Kim MK. J Anal .Toxicol. 1995; 19: 120-123.
- Kim JY, In MK, Paeng K, Chung BC. Chromatographia. 2004; 59; 219-226.
- Tayyaba Shaikh, Ayman Nafady, Farah N. Talpur, Sirajuddin and Samia Siddiqui. J Sensors and Actuators B: Chemical. 2015; 211: 359-369.
- Yoe JH, Jones AL. Ind Eng. Chem Anal Edition. 1944; 16: 14.
- Job P. Camp. Rend. (Paris). 180 (1925) 928. Ann. Chim. (Paris). 9. (1928) 133. Ann Chim. (Paris), 6 (1936) 97.
- Inczedy J. Analytical application of complex equilibria, Ellis Horwood Ltd, England. 1976; 137.
- British Pharmacopoeia. Vol II, HMSO, International edition. Cambridge. 2009; 6317.
- United States Pharmacopia. Twentieth Review, the National Formulary, Nineteenth Review, the United States Pharmacopeial Convention, Rockville, MD. 2000.
- Dowdy S, Weardern S.”Statistics for Research”, Wiley. NY.1983.
- Miller JC, Miller JN. Significance Tests in Statistics for Analytical Chemistry, 3rd edition. Chap 3, Ellis Hardwood, Chichester.UK. 1993.
- Caia Li, Zhanga J, Wang X, Zhua RH, Yanga J, Zhanga Qi, Penga WX. Biomed. Chromatogr. 2011.
- Groenendaal D, Blom-Roosemalen MCM, Danhof M, de Lange ECM. J Chromatogr B. 2005; 822: 230-237.
- Pao L, Hsiong C, Hu O, Ho S. J Chromatogr. 2000; 746: 241-247.
- Quarry MA, Sebastian DS, Williams RC. Chromatographia. 1998; 515-522.
- Nicolle E, Michaut S, Serre-Debeauvais F, Bessard G, Chromatogr J. 663; 1995: 111-117.
- Li S, Youxuan X, Yun W, Shan W, Changjiu Z. J Chinese Mass Spect. Socit. 1995; 3: 63-68.
- Kintz P, Tracqui A, Mangin P. J Chromatogr. 1992; 579: 172-176.
- Dubé LM, Beaudoin N, Lalande M, McGilveray IJ, Chromatogr J. 1988; 427: 113-120.
- Wetzelsberger N, Lucker PW, Erking W. Arzneimittel Forschung. 1988; 38: 1768-1771.
- Lo MW, Juergens GP, Whitney CC. J Res Commun Chem Pathol Pharmacol. 1984; 43: 51-64.
- Nada F, Ahmed Galal A, Samar H, Hassan J. Electroanalytical Chemistry. 2019; 839; 48-58.
- Kalwar NH, Tunesi MM, Soomro RA, Soomro RA, Karakus S. J Journal of Electroanalytical Chemistry. 2017; 80715: 137-144.
- Fouladgar M. J Sensors and Actuators B: Chemical. 2016; 230: 456-462.