
Research Article
Austin J Anal Pharm Chem. 2021; 8(1): 1129.
Development and Validation of HPLC Method for Simultaneous Estimation of Reduced and Oxidized Glutathione in Bulk Pharmaceutical Formulation
Singh N, Akhtar MJ and Anchliya A*
Department of Pharmaceutical Chemistry & Analysis, ISF College of Pharmacy, India
*Corresponding author: Anchliya A, Department of Pharmaceutical Chemistry & Analysis, ISF College of Pharmacy, Ferozepur GT Road, Moga-142001, Punjab, India
Received: August 07, 2020; Accepted: February 24, 2021; Published: March 03, 2021
Abstract
The objective of this study was the development, optimization, and validation of a RP-HPLC method for the quantification of reduced glutathione (GSH) and oxidized glutathione (GSSG) in pharmaceutical formulations The separation utilized a C18 column at room temperature with absorption wavelength 210nm. The mobile phase was an isocratic flow of a 95:5 (v/v) mixture of 25mM phosphate buffer (pH 2.7) and methanol with flow rate at 1.0 mL/min. Validation of the method assessed with the methods ability in seven categories: linearity, range, limit of detection, limit of quantification, accuracy, precision, and selectivity. The method show an acceptable degree of linearity with r²=0.9994 and 0.999 over a concentration range of 10-200 μg/mL for GSH and GSSG respectively. The detection limit and quantification limit for GSH 20.7μg/mL and 69.24μg/mL and for GSSG 17.22μg/mL and 57.42μg/mL respectively. The percent recovery of the method was 99.98-100.93 %. Following validation, the method was employed in the determination of glutathione in pharmaceutical formulations in the form of a liposome. The proposed method offers a simple, accurate, and inexpensive way to quantify reduced glutathione.
Keywords: Glutathione; Oxidized glutathione; RP-HPLC; Calibration curve; LOD; LOQ
Introduction
Glutathione (GSH) is an antioxidants biosynthesized in human, animal, plant and microbial cells such as fungi and bacteria. The chemical name of GSH and GSSG is l-glutamyl-l-cysteinylglycine [1-4]. This biological tripeptide plays vibrant roles in numerous physiological processes such as DNA synthesis protein synthesis cell membrane stabilization amino acid transportation and xenobiotic detoxification [5-10]. Glutathione acts as an electron donor through reduction of the thiol group in the cysteine moiety and the formation of the oxidized disulfide form (GSSG) [11]. After that, the enzyme, GSH reductase, converts GSSG back to GSH using Nicotinamide Adenine Dinucleotide Phosphate Hydrogen (NADPH) as a cofactor. Thus, the molar ratio of GSH/GSSG is an important measure of the cellular oxidative stress. The low GSH/GSSG ratio is an important biomarker in a number of human disorders such as cataract, cancer, parkinsonism, diabetes, renal failure, pneumonia, Alzheimer’s disease, lead toxicity, cystic fibrosis, dystrophic skin fibroblasts, neurodegenerative disorders, and liver and human immunodeficiency virus infections [12-16]. Determination of GSH is challenging because of the following reasons: (i) GSH can be easily autoxidized to GSSG, which results in a lower GSH/GSSG ratio and the lack of good UV/Vis chromophore or native [17]. The number of publications for determination of GSH by different methods have been reported which include HPLC, Electrochemical methods, Capillary electrophoresis, Spectroscopic probes and sensors [14,18-21]. But no methods have been reported for simultaneous determination of reduced (GSH) and oxidized glutathione (GSSG) in pharmaceutical formulation. For this purpose, liposomal preparations were used. Hence, a successful attempt has been made to estimate GSH, GSSG simultaneously by RP-HPLC method in the present work. The proposed methods were optimized and validated as per ICH guidelines [16,22-24].
Materials and Methods
Chemicals and reagents
Reduced and oxidized glutathione reference substance was kindly provided by Kyowa Hakko Bio Co. (Tokyo, Japan). Methanol HPLC grade were purchased from Rankem (RFCL limited) New Delhi. Potassium dihydrogen phosphate and orthophosphoric acid (85%, w/v) were obtained from Delhi (India). The 0.45μm nylon filters were purchased from Advanced Micro Devices Pvt. Ltd. Chandigarh, India. Double distilled water was used throughout the experiment which was generated in-house.
Apparatus and chromatographic conditions
The chromatographic analysis was performed on HPLC system of WATERS (Milford, USA) composed of 515 HPLC pump as a solvent delivery system equipped with Rheodyne injection valve with a 20μL loop, WATERS 2998 Photo Diode Array Detector (PDA) detector and separation was performed on C18 column (4.6×150 mm, 5μm i.d.) at 25°C column temperature. Chromatographic data were recorded and processed using EMPOWER-2 software.
Optimization of chromatographic conditions
In order to achieve the best separation of the peaks, we have changed different chromatographic conditions. Suitable conditions for the method validation were selected. Various trials have been done in the above optimized parameters individually or in combination. To achieve the proper separation, various conditions were applied, which include mobile phase composition i.e. 25mM potassium dihydrogen Phosphate buffer: methanol in the different ratio, at different pH and flow rate. For stability of reduced and oxidized glutathione stability study was also done to stabilize the oxidized glutathione throughout the study. The work was carried out in an air-conditioned room maintained at temperature 25±2°C and total run time was 10 min.
Chromatographic conditions: The composition of potassium dihydrogen phosphate buffer pH 2.7: methanol (30:45 v/v) has been found to be satisfactory for the complete separation of individual compounds. Before use, the mobile phase was filtered through nylon 0.45μm membrane filter and also it was degassed for 30 min. The standard stock solutions (1000μg/mL) of each drug were prepared. To get the concentration of Stock solution for GSH, GSSG (25mg each) were weighed accurately and separately transferred into individual 25mL volumetric flasks. The 0.1% EDTA solution added up to 70ml and the solution were sonicated to get the maximum dissolution of the drugs. 0.1% EDTA solution was used to stabilize the GSSG. Further 0.1% EDTA solution was used to made up the volume up to the mark 25mL. From these stock solutions, further dilutions were made in the concentration range of 10-200μg/mL of GSH, GSSG respectively. A volume of 20μL of each sample was used for injection.
Selection of wavelength: The standard stock solution 1mg/mL of GSH, GSSG were prepared separately by weighing 25mg drug samples and transferred to a 25mL volumetric flask separately and volume made up to mark with 0.1% EDTA solution. From this solution, 0.1mL was transferred to 10 mL volumetric flask and volume made up to the mark with the 0.1% EDTA solution. The resulting solutions were scanned over the UV range (200-400), maximum absorbance was found at λmax 210nm for both drugs (overlain spectra).
Preparation of buffer: The 0.025M potassium dihydrogen phosphate buffer (pH 2.7) was used for method development. Buffer was prepared by dissolving 3.46g of sodium dihydrogen phosphate with Mili-Q water to 1000mL. The pH was adjusted with orthophosphoric acid using pH meter. The prepared buffer was passed through 0.45μm membrane filter.
Preparation of mobile phase: Mobile phase was prepared by mixing 0.025M sodium dihydrogen phosphate buffer (2.7) and methanol (HPLC grade) in 95:5 (v/v) proportions. Then the mixture was sonicated for 30 min prior to use.
Preparation of diluent solution: 0.1% EDTA solution was prepared by adding 0.5gm of EDTA in 500mL volumetric flask and adds 100ml of mobile phase heat the solution to completely dissolve the EDTA and makeup the volume up to 500mL with mobile phase.
Preparation of stock solutions and test solutions: Stock solution was prepared by dissolving GSH, GSSG (25mg each) that were weighed accurately and separately transferred into 25mL volumetric flasks. Similarly, the binary mixture of GSH, GSSG was also prepared as that of stock solutions by dissolving 25mg of both drugs in 25mL volumetric flasks. Add 0.1% EDTA solution, then sonicated for 10 min and diluted up to 25mL. A series of solutions were prepared in the concentration range of 10-200 μg/mL of both GSH, GSSG. Solution are filtered through 0.45μm syringe filter All.
Preparation of test solutions: Sample solution was prepared by dissolving 1ml of reduced glutathione liposomal formulation into 10 ml diluent and shakes the sample for mixing after that the sample was filtered through 0.45μm syringe filter. The sample was then used for replicate analysis.
Preparation of calibration curve: The calibration curve was prepared by injecting concentration of 10-200 μg/mL of GSH and GSSG, and ternary mixture solutions manually in triplicate to the HPLC system at detection wavelength of 210 nm. Mean of n=6 determinations was plotted as the standard curve. The calibration curve was tested and validated with inter day and intraday measurements.
RP-HPLC method
For marketed formulation, an assay was performed to check the purity of each drug in the formulation and percentage purity of the drugs was also calculated. The 100μL of standard and sample solution were injected by knowing the peak area of GSH and GSSG the amount of drugs in sample were calculated.
Method validation: Validation of the new simultaneous spectrophotometry and RP-HPLC methods were carried out as recommended by the International Conference on Harmonization 24 for all the validation parameters including linearity, accuracy, Limit of Detection (LOD), Limit of Quantification (LOQ), precision, specificity, and robustness.
Specificity: Specificity is the competency of an analytical method to measure the analyte free from interferences due to other components. The procedures used to substantiate specificity will depend on the intended objective of the analytical procedure. Specificity of the method was assessed by comparing the chromatograms obtained from standard drugs with the chromatogram that obtained from tablet solutions (formulation).
Linearity: The linearity of the method was determined by analyzing several aliquots of standard solution of GSH and GSSG. For RP-HPLC method, linear correlations were obtained between peak area and concentration for GSH and GSSG in the ranges of 10-200 μg/mL, respectively.
Accuracy: The accuracy of the method was determined by recovery studies using the standard addition method. Pre analyzed samples were spiked with standard drugs GSH, GSSG at three different concentration levels, i.e. 80, 100 and 120%, and the mixtures were reanalyzed by the proposed method in triplicates. Data obtained was analyzed for percent recovery. For RP-HPLC method, known amount of the standard solution (80, 100 and 120μg/mL) of individual drug were added to a pre-analyzed sample solution of GSH (100μg/ mL) GSSG (100μg/mL).
Precision: Precision of an analytical method demonstrate the closeness of agreement between a set of measurements that obtained from the multiple sampling of the same sample under normal operational conditions. The measurement of precision was further determined by repeatability intermediate precision and reproducibility. Moreover precision can be reported as standard deviation or relative standard deviation for a statistically significant number of replicate measurements.
Limit of detection and limit of quantification: Limit of Detection (LOD) and Limit of Quantification (LOQ) are two important parameters that must be validated during analytical method development and validation process. The LOD and LOQ are the well-known performance characteristics that express the smallest concentration of an analyte that can be calculated by an analytical procedure. The Limit of Detection (LOD) is defined as the lowest concentration of an analyte that can be detected but not exactly quantitated as per its exact concentration level. Whereas, the Limit of Quantification (LOQ) is defined as the lowest concentration of analyte that can be measured with acceptable accuracy and precision under specific test conditions. Generally, a typical signal-to-noise ratio found to be 3:1 and 10:1 for LOD and LOQ, respectively. Hence, the LOD and LOQ were calculated as
LOD = 3.3×s/S
LOQ =10×s/S
Where,
s=Standard deviation of the lowest standard concentration;
S=Slope of the standard curve.
Result and Discussion
RP-HPLC method
Validation of the proposed method:
a) Specificity
The retention time for GSH and GSSG was 4.52 min, 6.73 min respectively. The chromatograms have been shown in shown in (Figure 1&2).
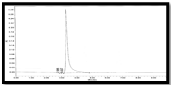
Figure 1: RP-HPLC Blank chromatogram.
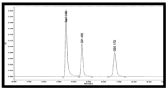
Figure 2: RP-HPLC chromatogram of GSH and GSSG (100ppm).
b) Linearity
Linear correlations were obtained in concentration range of 10- 200 μg/mL with the correlation coefficient of 0.9992 and 0.0999 for GSH and GSSG respectively. Calibration curve was plotted using AUC versus of standard solution as shown in (Figure 1a&1b). Peak areas were recorded for all the peaks. The values of the linearity data are shown in (Table 1).
A six-point calibration curve was constructed with working standards and was found linear (r²>0.999) for each of the analyte over their calibration ranges. The slopes were calculated using the plot of drug concentration versus area of the chromatogram. Linearity was found to be quite satisfactory and reproducible as shown in (Figure 3&4). The Overlay spectra of GSH and GSSG are depicted in (Figure 5).
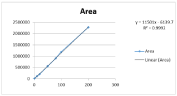
Figure 3: Linearity curves of GSH by HPLC Method.
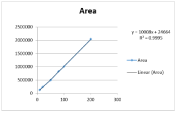
Figure 4: Linearity curves of GSSG by HPLC method.
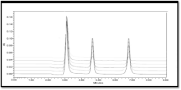
Figure 5: Overly spectra of GSH and GSSG.
Graphical Abstract: Graphical Abstract
c) Precision
Standard dilutions were prepared and three replicates of each dilution were analyzed on same day for repeatability. The values of the RP-HPLC precision studies are shown in (Table 1).
Validation parameters
GSH
GSSG
Absorption maxima
210
210
Linearity range
10-200
10-200
Coefficient of determination (R2)
0.999
0.999
Regression equation
y=10008x +24664
y=12122x -30408
Slop (b)
10008
12122
Intercept (a)
24664
30408
Limit of detection
20.77475
17.226523
Limit of quantification
69.249165
57.421745
Table 1: Resulting validation parameters for HPLC method.
d) LOD and LOQ
For the RP-HPLC method, LOD was found to be 20.77 and 17.22μg/mL, and LOQ was found to be 69.24 and 57.421745μg/mL for GSH, GSSG respectively. The values of the LOD and LOQ in the RP-HPLC method are shown in (Table 1).
e) Accuracy as recovery
The accuracy of the method was determined by recovery studies. The Results of the recovery study were found to be within the acceptance criteria 100±10 %, indicating a good degree of sensitivity of the method towards detection of analytes in sample. The values of the accuracy studies for the RP-HPLC methods are shown in (Table 2).
Amt. added (µg/mL)
%Recovery
GSHG
SSG
GSH
GSSG
80
80
99.99
99.98
100
100
100.91
100.13
120
120
99.92
100.12
Table 2: Recovery studies of GSH, GSSG by HPLC methods.
f) Assay of marketed formulation
For marketed formulation, an assay was performed to check the purity of each drug in the formulation and percentage purity of the drugs was calculated. Percentage estimation for and was found to be and respectively. Results are shown in (Table 3).
Drug name
Drug claim (mg)
Amount found (mg)
%Estimation
Liposomal reduced glutathione
100
108.5
108.50%
Table 3: Assay of GSH by HPLC methods.
Conclusion
The proposed method was first developed and optimized for simultaneous estimation for reduced and oxidized glutathione in pharmaceutical formulation. The proposed method analytically tested different parameters. Each parameter was evaluated, and once all parameters had been tested, the method was concluded and subjected to repeat testing in order to validate the procedure. Validation evaluated the linearity, range, limit of detection, limit of quantification, accuracy, precision, and specificity of the proposed analytical method. After validation the method was applied for the analysis of pharmaceutical preparations. The proposed validated RPHPLC method for the analysis of GSH and GSSG achieved acceptable levels of precision, linearity, sensitivity, reproducibility, selectivity, and accuracy. Likewise, all of the system suitability parameters are within the acceptable range, making this an acceptable method for quantifying GSH and GSSG by RP-HPLC.
Acknowledgement
The authors are thankful to the to Mr. Praveen Garg, Chairman and Prof Dr. GD Gupta, Director cum Principal, ISF College of Pharmacy, Moga, Punjab for his continuous support and encouragement.
References
- Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Molecular aspects of medicine. 2009; 30: 1-12.
- Liedschulte V, Wachter A, Zhigang A, Rausch T. Exploiting plants for glutathione (GSH) production: uncoupling GSH synthesis from cellular controls results in unprecedented GSH accumulation. Plant Biotechnology Journal. 2010; 8: 807-820.
- Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Critical reviews in clinical laboratory sciences. 2009; 46: 241-281.
- Begara-Morales JC, Sanchez-Calvo B, Chaki M, Valderrama R, Mata-Perez C, Padilla MN, et al. Antioxidant systems are regulated by nitric oxidemediated post-translational modifications (NO-PTMs). Frontiers in Plant Science. 2016; 7: 152.
- Berenbaum MR, Johnson RM. Xenobiotic detoxification pathways in honey bees. Current opinion in insect science. 2015; 10: 51-58.
- Navarro J, Obrador E, Carretero J, Petschen I, Avino J, Perez P, et al. Changes in glutathione status and the antioxidant system in blood and in cancer cells associate with tumour growth in vivo. Free Radical Biology and Medicine. 1999; 26: 410-418.
- Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP. Inhibition of influenza infection by glutathione. Free Radical Biology and Medicine. 2003; 34: 928-936.
- Droge W, Holm E. Role of cysteine and glutathione in HIV infection and other diseases associated with muscle wasting and immunological dysfunction. The FASEB journal. 1997; 11: 1077-1089.
- Hercbergs A, Brok-Simoni F, Holtzman F, Bar-Am J, Brenner H, Leith J. Erythrocyte glutathione and tumour response to chemotherapy. The Lancet. 1992; 339: 1074-1076.
- Alisik M, Neselioglu S, Erel O. A colorimetric method to measure oxidized, reduced and total glutathione levels in erythrocytes. Journal of Laboratory Medicine. 2019; 43: 269-277.
- Gennaro M, Abrigo C. Separation of reduced and oxidized glutathione in a pharmaceutical preparation by ion-interaction reversed-phase HPLC. Journal of Pharmaceutical and Biomedical Analysis. 1992; 10: 61-65.
- Raggi M, Nobile L, Giovannini A. Spectrophotometric determination of glutathione and of its oxidation product in pharmaceutical dosage forms. Journal of Pharmaceutical and Biomedical Analysis. 1991; 9: 1037-1040.
- Di Pietra A, Gotti R, Bonazzi D, Andrisano V, Cavrini V. HPLC determination of glutathione and L-cysteine in pharmaceuticals after derivatization with ethacrynic acid. Journal of Pharmaceutical and Biomedical Analysis. 1994; 12: 91-98.
- Hamad A, Elshahawy M, Negm A, Mansour FR. Analytical methods for determination of glutathione and glutathione disulfide in pharmaceuticals and biological fluids. Reviews in Analytical Chemistry. 2019; 38.
- Sutariya V, Wehrung D, Geldenhuys WJ. Development and validation of a novel RP-HPLC method for the analysis of reduced glutathione. Journal of chromatographic science. 2012; 50: 271-276.
- Guideline IHT. Validation of analytical procedures: text and methodology. Q2 (R1). 2005; 1: 1-15.
- Jones DP, Carlson JL, Samiec PS, Sternberg Jr P, Mody Jr VC, Reed RL, et al. Glutathione measurement in human plasma: evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clinica chimica acta. 1998; 275: 175-184.
- Reginald-Opara JN, Svirskis D, O’Carroll SJ, Sreebhavan S, Dean JM, Wu Z. Optimisation of glutathione conjugation to liposomes quantified with a validated HPLC assay. International journal of pharmaceutics. 2019; 567: 118451.
- Sadowska-Bartosz IA, Furmaniak P, Bieszczad-Bedrejczuk E, Bartosz G, Glowacki R. Developmental changes in the levels and redox potentials of main hemolymph thiols/disulfides in the Jamaican field cricket Gryllus assimilis. Acta Biochimica Polonica. 2017; 64: 503-506.
- Zhang W, Li P, Geng Q, Duan Y, Guo M, Cao Y. Simultaneous determination of glutathione, cysteine, homocysteine, and cysteinylglycine in biological fluids by ion-pairing high-performance liquid chromatography coupled with precolumn derivatization. Journal of agricultural and food chemistry. 2014; 62: 5845-5852.
- Robin S, Leveque N, Courderot-Masuyer C, Humbert P. LC-MS determination of oxidized and reduced glutathione in human dermis: a microdialysis study. Journal of Chromatography B. 2011; 879: 3599-3606.
- Turner A. Introduction to high performance liquid chromatography by RJ Hamilton and PA Sewell. pp 248. Chapman & Hall, London. 1982.£ 13.50 ISBN 0-412-23430-0. Biochemical Education. 1983; 11: 45-46.
- Meyer VR. Practical high-performance liquid chromatography: John Wiley & Sons. 2013.
- Guideline IHT. Validation of Analytical Procedures: Text and Methodology, Q2 (R1) Geneva, 2005. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2013.