
Research Article
Austin J Anal Pharm Chem. 2022; 9(1): 1141.
Design and Synthesis of New Compounds Derived from Phenyl Hydrazine and Different Aldehydes as Anticancer Agents
Salem M¹*, Ayyad R², Sakr H² and Gaafer A³
¹Department of Medicinal Chemistry, Faculty of Pharmacy, Sinai University, Egypt
²Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Al-Azhar University, Egypt
³Medical Services Sector, Interior Ministry, Egypt
*Corresponding author: Mo’men Salem, Department of Medicinal Chemistry, Faculty of Pharmacy, Sinai University, Egypt
Received: February 21, 2022; Accepted: March 17, 2022; Published: March 24, 2022
Abstract
In this work we synthesized new derivatives from Phenyl Hydrazine and series of different Aldehydes (derivatives of benzylidenes). The synthesized compounds contain different aromatic Aldehydes which attached by Benzene ring via Hydrazine moiety. These derivatives were characterized by TLC, melting points, Infrared Red, Proton Nuclear Magnetic Resonance, Carbon Thirteen Nuclear Magnetic Resonance and Mass Spectroscopy. Finally, these synthesized derivatives were tested for antiproliferative activity against multiple normal and cancerous cell lines, HepG2 (Liver Cancer) and MCF-7 (Breast Cancer) cell lines were used for cytotoxic assay.
Keywords: Phenyl hydrazine; Aromatic aldehydes; Benzylidene synthesis; Cytotoxic assay; Anticancer; HepG2; MCF-7
Introduction
Cancer is a public health menace. The disease is of a great concern to both developed and developing countries due to its high morbidity and mortality. In many countries, it has become second largest killer after cardiovascular disease [1]. In 2012, there were 14 million new cases and 8.2 million deaths [1]. Among men, lung cancer was the most predominant, while among women, it was breast cancer. It was reported that there were 24 million cancer cases annually and 14.6 million annual deaths by the end of 2015 [2]. These troubling figures compel policy makers and the researchers to combat this disease. Cancer is a collection of different life-threatening diseases characterized by uncontrolled growth of cells leading to invasion of surrounding tissue and often spreading to other parts of the body [3]. When it comes to understanding and controlling cancer scientists are now working from a position of strength because a foundation of knowledge about cancer has been built over the past 50 years. There is an urgent need for novel effective drug regimens for the treatment of cancer because the current chemotherapy suffers from a slim therapeutic index, with significant toxicity from effective drug doses or tumor recurrence at low drug doses. The new anticancer chemotherapeutic agents search continues to be an active area of research at many companies and research centers [3,4]. Searching for new anticancer agents having heterocyclic nucleus continues worldwide at various laboratories [5-7]. So these organic compounds synthesized and tested as anticancer drugs. Synthesized compounds have benzene ring attached to five or six membered rings (Benzimidazole) or (Phthalazine, Quinazoline, Quinoxalines). In this work we aimed the synthesis of organic compounds formed of benzene ring attached by Hydrazine moiety which is two nitrogen atoms but not fused in the ring as Phthalazines, Quinazolines, Quinoxalines or Benzimidazoles. These new compounds have two nitrogen atoms in side chain as a bridge between benzene ring and aromatic aldehydes [8-44].
Materials
Reagents
All solvents and reagents were obtained from commercial sources and were used without further purification except Glacial Acetic acid and Petroleum Ether (PE). Phenyl Hydrazine was purchased from Sigma Aldrich (Cairo, Egypt). Series of Aromatic Aldehydes were acquired from Sigma Aldrich (Cairo, Egypt). Absolute Ethanol, Ehanol 95%, Glacial Acetic Acid, Ethyl Acetate, Petroleum Ether and Chloroform were purchased from Piochem (Cairo, Egypt). Distilled water was used for the experiments.
Instruments
Progress of chemical reactions was observed using TLC (Merck, silica gel plates 60 F254) and visualized using a UV-Visspectrometer at 254nm. Melting points were determined by Mel-Temp apparatus. NMR spectra were performed in Chloroform(1) (7.26ppm), with trimethyl silane as an internal standard, using Bruker Avance 500 spectrometer at ambient temperature, at drug discovery unit, Faculty of Pharmacy, Ain Shams University (ASU, Cairo, Egypt). All chemical shifts were expressed in parts per million (d), and coupling constants (J) in Hz. FTIR spectra were recorded using KBr pellets on a model 883 double beam infrared spectrophotometer Bruker in 200- 4000cm-1, at drug discovery unit, Faculty of Pharmacy, Ain Shams University (ASU, Cairo, Egypt). MS spectra were recorded using a Bruker Esquire 2000 by APC or ES ionization, at drug discovery unit, Faculty of Pharmacy, Ain Shams University (ASU, Cairo, Egypt).
Cell culture: HepG2, MCF-7
Cell line was obtained from Nawah Scientific Inc., (Mokatam, Cairo, Egypt). Cells were maintained in DMEM media supplemented with 100mg/mL of streptomycin, 100nits/mL of penicillin and 10% of heat-inactivated fetal bovine serum in humidified, 5% (v/v) CO2 atmosphere at 37°C [45,46].
Cytotoxicity assay: Hep G2, MCF-7
Cell viability was assessed by SRB assay. Aliquots of 100μL cell suspension (5x10^3 cells) were in 96-well plates and incubated in complete media for 24h. Cells were treated with another aliquot of 100μL media containing drugs at various concentrations. After 72h of drug exposure, cells were fixed by replacing media with 150μL of 10% TCA and incubated at 4°C for 1h. The TCA solution was removed, and the cells were washed 5 times with distilled water. Aliquots of 70μL SRB solution (0.4%w/v) were added and incubated in a dark place at room temperature for 10min. Plates were washed 3 times with 1% acetic acid and allowed to air-dry overnight. Then, 150μL of TRIS (10mM) was added to dissolve protein-bound SRB stain; the absorbance was measured at 540nm using a BMG LABTECH®- FLUOstar Omega microplate reader (Ortenberg, Germany) [45,46].
Chemistry and Scheme
Scheme
See Figure 1.

Figure 1: Reagents and conditions: (i) Series of Aromatic Aldehydes, Refluxing Glacial Acetic Acid, 135°C, 1-16h.
Procedure and synthesis of Compounds 3-13
Compound 3: (E)-1-benzylidene-2-phenylhydrazine: Equimolar mixture of Phenyl Hydrazine (20ml, 22gm, 0.202mole) and Benzaldehyde (20.5ml, 21.53gm, 0.202mole) are stirred together in refluxing Glacial Acetic acid for 1hour, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. Precipitate was obtained from organic layer then water was added and more precipitate was retrieved. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in good yield 70%. m.p = 154-156 °C.
IR: 688.75, 747.51cm-1 (aromatic, bending), 880.40cm-1 (N-H, overtone), 1064.45cm-1 (C-N), 1518cm-1 (N-H, bending), 1590cm-1 (C=C, aromatic), 2450cm-1 (aromatic, overtone), 3090cm-1 (C-H, aromatic) and 3300 cm-1 (N-H, stretching).
¹HNMR (400MHz, CDCl3): d6.90-7.50ppm (m, aromatic protons), 7.65ppm (s, -CH-) and 10.3ppm (s,-NH-).
13CNMR (100MHz, CDCl3): dC1 (144.5ppm), C2 (117ppm), C3 (114ppm), C4 (137ppm), C5 (114ppm), C6 (117ppm), C7 (146ppm), C1 (147.5ppm), C2 (115ppm), C3 (130ppm), C4 (125ppm), C5 (130ppm) and C6(115 ppm).
Compound 4: (E)-1-(4-methoxybenzylidene)-2- phenylhydrazine: Equimolar mixture of Phenyl Hydrazine (4ml, 4.47gm, 0.042mole) and 4-Methoxybenzaldehyde (5ml, 5.6gm, 0.042mole) are stirred together in refluxing Glacial Acetic acid for 1 and half hours, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. Precipitate was obtained from organic layer then water was added and more precipitate was retrieved. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in good yield 82.5%. m.p = 128-130 °C.
¹HNMR (400MHz, CDCl3): d3.86ppm (s,-CH3-), 6.85-7.35ppm (m, aromatic protons), 7.65ppm (s,-CH-) and 9.9ppm (s,-NH-).
13CNMR(100MHz, CDCl3): dC1 (54.3ppm), C2 (158.9ppm), C3 (113.6ppm), C4 (129.8ppm), C5 (124.8ppm), C6 (129.8), C7 (113.6ppm), C8 (143.8ppm), C1 (145.2ppm), C2 (112.2ppm), C3 (129.5ppm), C4 (128.8ppm), C5 (129.5ppm) and C6 (112.2ppm).
Compound 5: (E)-1-(2-chlorobenzylidene)-2-phenylhydrazine: Equimolar mixture of Phenyl Hydrazine (4.4ml, 4.8gm, 0.044mole) and 2-Chlorobenzaldehyde (5ml, 6.24gm, 0.044mole) are stirred together in refluxing Glacial Acetic acid for 15 hours, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. Precipitate was obtained from organic layer then water was added and more precipitate was retrieved. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in good yield 73%. m.p = 129-131 °C.
¹HNMR (400MHz, CDCl3): d6.75-7.75ppm (m, aromatic protons), 7.85ppm (s,-CH-) and 10.5ppm (s,-NH-).
MS Spectroscopy: m/z: 230.06 (100.0%), (M+1) 231.05 (87.9%), (M+2) 229.05 (12.1%).
Compound 6: 4-((2-phenylhydrazono) methyl)phenol: Equimolar mixture of Phenyl Hydrazine (4ml, 4.43gm, 0.041mole) and 4-Hydroxybenzaldehyde (5gm, 0.041mole) are stirred together in refluxing Glacial Acetic acid for 1 hour1, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. Precipitate was obtained from organic layer then water was added and more precipitate was retrieved. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in good yield 86 %. m.p = 178-181 °C.
IR: 690.59, 743.83cm-1 (aromatic, bending), 884.73cm-1 (N-H, overtone), 1098.33cm-1 (C-N), 1504cm-1 (N-H, bending), 1596.49cm-1 (C=C, aromatic), 1700cm-1 (C=N), 3045cm-1 (C-H, aromatic), 3290cm-1 (N-H, stretching) and 2900-3625cm-1 (OH).
¹HNMR(400MHz, CDCl3): d6.85-7.55ppm (m, aromatic protons), 7.7ppm (s, -CH-), 7.85ppm (s, -OH) and 9.88ppm (s, -NH-).
13CNMR(100 MHz, CDCl3): dC1 (158.82ppm), C2 (117.56ppm), C3 (130.8ppm), C4 (125.4ppm), C5 (130.8ppm), C6 (117.56), C7 (140.7ppm), C1 (146.22ppm), C2 (113.9ppm), C3 (129.5ppm), C4 (122.8ppm), C5 (129.5ppm) and C6 (113.9ppm).
Compound 7: 4-((2-phenylhydrazono) methyl)pyridine: Equimolar mixture of Phenyl Hydrazine (10ml, 11gm, 0.102mole) and 4-Pyridinecarldehyde (9.6ml, 10.88gm, 0.102mole) are stirred together in refluxing Ethanol for 1hour, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. Precipitate was obtained from organic layer then water was added and more precipitate was retrieved.
Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in good yield 73%. m.p = 179-181 °C.
¹HNMR (400MHz, CDCl3): d6.90-8.55ppm (m, aromatic protons), 7.60ppm (s, -CH-) and 8.15 (s, -NH-).
13CNMR (100MHz, CDCl3): dC2 (149.98ppm), C3 (120.13ppm), C4 (143.47ppm), C5 (120.13ppm), C6 (149.98ppm), C7 (142.84ppm), C1 (133.55ppm), C2 (113.09ppm), C3 (129.42ppm), C4 (121.13ppm), C5 (129.42ppm) and C6 (113.09ppm).
Compound 8: (E)-1-(4-nitrobenzylidene)-2-phenylhydrazine: Equal mixture of Phenyl Hydrazine (1ml, 1.016gm, 0.009mole) and 4-Nitrobenzaldehyde (1.42gm, 0.009mole) are stirred together in refluxing Glacial Acetic acid for 6hours, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. No precipitate was obtained from acetic acid layer then equal amounts of water and ethanol 95% wereadded to obtain the product. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in relatively low yield 32.2%. m.p = 110-112 °C.
¹HNMR (400MHz, CDCl3): d6.80-7.40ppm (m, aromatic protons), 7.55ppm (s, -CH-) and 9.88ppm (s, -NH-).
13CNMR (100 MHz, CDCl3): dC1 (147.18ppm), C2 (119.06ppm), C3 (119.84ppm), C4 (144.93ppm), C5 (119.84ppm), C6 (119.06ppm), C7 (137.29ppm), C1 (145.85ppm), C2 (111.66ppm), C3 (129.28ppm), C4 (112.71ppm), C5 (129.28ppm) and C6 (111.66ppm).
Compound 9: (E)-1-(furan-2-ylmethylene)-2-phenylhydrazine: Equal amounts of Phenyl Hydrazine (5ml, 5.5gm, 0.05mole) and Furan-2-carbaldehyde (4.2ml, 4.88gm, 0.05mole) are stirred together in refluxing Glacial Acetic acid for 10hours, TLC was made to monitor the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. No precipitate was obtained from acetic acid layer. Water was added to obtain the product. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in average yield 65%. m.p = 113-115 °C.
IR: 692.95, 743.06cm-1 (aromatic, bending), 818.48cm-1 (N-H, overtone), 1153.57cm-1 (C-N), 1342.30cm-1(C-O), 1602.35 cm-1(C=C, aromatic), 1604cm-1(N-H, bending), 1655cm-1 (C=N), 2025cm-1 (CH, aromatic overtone), 3090cm-1 (C-H, aromatic) and 3317.56cm-1 (N-H, stretching).
¹HNMR (400MHz, CDCl3): d6.85-7.55ppm (m, aromatic protons), 7.60ppm (s, -CH-) and 9.75ppm (s, -NH-).
13CNMR (100MHz, CDCl3): dC2 (144.36ppm), C3 (112.89ppm), C4 (120.46ppm), C5 (150.55ppm), C6 (142.72ppm), C1 (143ppm), C2 (112.96ppm), C3 (129.31ppm), C4 (127.83ppm), C5 (129.31ppm) and C6 (112.96ppm).
Compound 10: (E)-1-phenyl-2-((E)-3-phenylallylidene) hydrazine: Equimolar mixture of Phenyl Hydrazine (5ml, 5.5gm, 0.05mole) and Cinnamaldehyde (6.4ml, 6.72gm, 0.05mole) are stirred together in refluxing Glacial Acetic acid for 1 hours, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. Precipitate was obtained from acetic acid layer; water was added to obtain more of the product. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in high yield 80.5%. m.p = 150-152 °C.
¹HNMR (400 MHz, CDCl3): d6.75ppm (t,-CH-), 7.05ppm (d,- CH-), 6.85-7.50ppm (m, aromatic protons), 7.55ppm (s,-CH-) and 9.75ppm (s, -NH-).
13CNMR (100 MHz, CDCl3): dC1 (132.5ppm), C2 (130ppm), C3 (127ppm), C4 (125ppm), C5 (127ppm), C6 (130ppm), C7 (134ppm), C8 (123ppm), C9 (140ppm), C1 (145ppm), C2 (118ppm), C3 (129ppm), C4 (122ppm), C5 (129ppm) and C6 (118ppm).
Compound 11: (E)-1-(4-chlorobenzylidene)-2- phenylhydrazine: Equimolar mixture of Phenyl Hydrazine (0.85ml, 0.92gm, 0.0085mole) and 4-Chlorobenzaldehyde (1.2gm, 0.0085mole) are stirred together in refluxing Glacial Acetic acid for 5 hours, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. Precipitate was obtained from acetic acid layer; water was added to obtain more of the product. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in high yield 80.1%. m.p = 119-121 °C.
IR: 691.09, 746.28cm-1 (mono-sub.), 819.32 cm-1 (para-di-sub.) (aromatic, bending), 882.19 cm-1 (N-H, overtone), 1133.08 cm-1 (CN), 1518.02 cm-1 (N-H, bending), 1598.38 cm-1 (C=C, aromatic), 1620.02cm-1 (C=N), 2000 cm-1 (C=C, aromatic), 3000 cm-1 (C-H, aromatic) and 3310.61cm-1 (N-H, stretching).
¹HNMR (400 MHz, CDCl3): d6.95-7.50ppm (m, aromatic protons), 7.90ppm (s,-CH-) and 10.10ppm (s, -NH-).
13CNMR (100MHz, CDCl3): dC1 (134.5ppm), C2 (130.2ppm), C3 (132.3ppm), C4 (136.9ppm), C5 (132.3 ppm), C6 (130.2ppm), C7 (140.5ppm), C1 (144.8ppm), C2 (112ppm), C3 (129.7ppm), C4 (122.9ppm), C5 (129ppm) and C6 (112ppm).
Mass Spectroscopy: m/z: 230.06 (100.0%), (M+1) 231.10 (63.7%), (M+2) 229.05 (36.3%).
Compound 12: (E)-1-(4-bromobenzylidene)-2- phenylhydrazine: Equimolar mixture of Phenyl Hydrazine (0.28ml, 0.3gm, 0.0028mole) and 4-Bromobenzaldehyde (0.52gm, 0.0028mole) are stirred together in refluxing Glacial Acetic acid for 7 hours, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. No precipitate was obtained from the organic layer, water was added to quench the reaction and from which the product was obtained. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in relatively high yield 71%. m.p = 115-117 °C.
¹HNMR (400 MHz, CDCl3): d7.0-7.60ppm (m, aromatic protons), 7.98ppm (s,-CH-) and 9.85ppm (s, -NH-).
13CNMR (100 MHz, CDCl3): dC1 (129.3ppm), C2 (133.3ppm), C3 (131.5ppm), C4 (136.7ppm), C5 (131.5 ppm), C6 (133.3ppm), C7 (142.8ppm), C1 (145.6ppm), C2 (113.8ppm), C3 (128ppm), C4 (121.4ppm), C5 (128ppm) and C6 (113.8ppm).
Mass Spectroscopy: m/z: 276 (100.0%), (M+1) 278.95 (70 %), (M+2) 280.95 (30%).
Compound 13: 1,4-bis((2-phenylhydrazono) methyl)benzene: Equimolar mixture of Phenyl Hydrazine (2.93ml, 3.22gm, 0.029mole) and Terephthaldehyde (4gm, 0.029mole) are stirred together in refluxing Glacial Acetic acid for 1hour, TLC was made to ensure the completion of the reaction by system 2:1 Petroleum Ether: Ethyl Acetate. Precipitate was obtained from the organic layer, later on, water was added to quench the reaction and from which the product was obtained. Product was purified by crystallization in Absolute Ethanol. The desired product was obtained in average yield 62%. m.p = 220-22°C.
IR: 690.53, 743.71cm-1 (aromatic, bending), 885.30cm-1 (NH, overtone), 1130.68cm-1 (C-N), 1522.08cm-1 (N-H, bending), 1588.48cm-1(C=C, aromatic), 1600.36cm-1 (C=N), 1925.25cm-1 (C-H, aromatic overtone), 3075.25cm-1 (C-H, aromatic) and 3299.42cm-1 (N-H, stretching).
¹HNMR (400MHz, CDCl3): d6.95-7.90ppm (m, aromatic protons), 7.75ppm (s, -CH-), 10.03ppm (s,-NH-).
13CNMR (100MHz, CDCl3): dC1 (145ppm), C2 (115ppm), C3 (130ppm), C4 (122ppm), C5 (130ppm), C6 (115ppm), C7 (140ppm), C8 (136ppm), C9 (129ppm), C10 (129ppm), C11 (136ppm), C12 (129ppm), C11 (136ppm), C12 (129ppm), C13 (129ppm), C14 (140ppm), C15 (145ppm), C16 (115ppm), C17 (130ppm), C18 (122ppm), C19 (130ppm) and C20 (115ppm).
Results
Cytotoxicity results of MCF-7
See Figure 2, 3 and 4.

Figure 2: IC50 Value results of compounds 3-8 against MCF-7 Cell line.
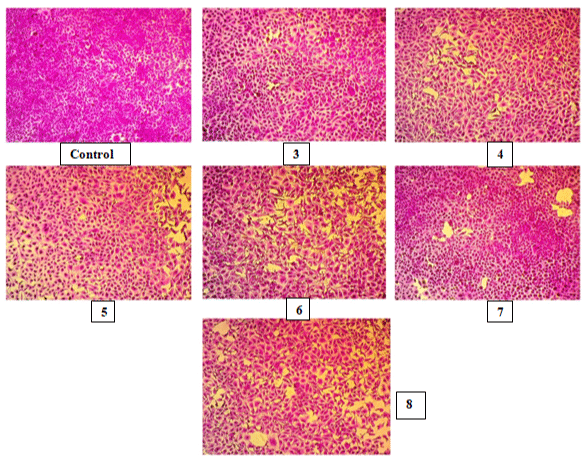
Figure 3: MCF-7 cell lines under microscopic examination of control and compounds (3-8) at 100μm concentration.
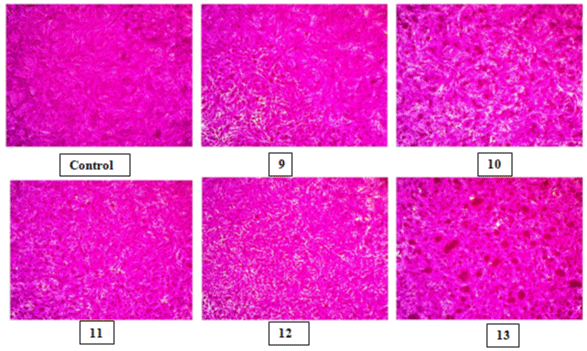
Figure 4: HepG2 cell lines under microscopic examination of control and compounds (9-13) at 100μm concentration.
Cytotoxicity results of HepG2
See Figure 5 and Table 1.

Figure 5: IC50 Value results of compounds 9-13 against HepG2 Cell line.
Compound
IC50
Cell Line Type
Standard Drug
IC50
3
51.18
MCF-7
Vincristine
0.82
4
61.18
MCF-7
Vincristine
0.82
5
49.19
MCF-7
Vincristine
0.82
6
55.99
MCF-7
Vincristine
0.82
7
100.09
MCF-7
Vincristine
0.82
8
45.39
MCF-7
Vincristine
0.82
9
169.84
HepG2
Doxorubicin
9.5
10
127.69
HepG2
Doxorubicin
9.5
11
163.26
HepG2
Doxorubicin
9.5
12
143.75
HepG2
Doxorubicin
9.5
13
555.66
HepG2
Doxorubicin
9.5
Table 1: Summary of the cytotoxic assay results of all compounds in two different cell lines.
Conclusion
From the above findings, we concluded that all tested compounds have potential antiproliferative activity on both cell lines which were tested. For MCF-7cell line, compound 8 was found to be the most potent compound in the group scoring 45.39μm IC50, compound 7 was the lowest in potency scoring 100.09μm IC50. For HepG2 cell line, compound 10was found to be the most potent compound among the other compounds scoring 127.69μm IC50and compound 13 was the lowest in potency in this group.
References
- Cancer: Factsheet No. 297. 2015.
- International Agency for Cancer Research, WHO, World Cancer Factsheet. 2014.
- Bridges AJ. Chemical inhibitors of protein kinases. Chem Rev. 2001; 101: 2541-2572.
- Wang JD, Miller K, Boschelli DH, Ye F, Wu B, Floyd MB, et al. Inhibitors of Src tyrosine kinase: The preparation and structure- activity relationship of 4-anilino-3-cyanoquinolines and 4-anilinoquinazolines. Bioorg Med Chem Lett. 2000; 10: 2477-2480.
- Cai SX. Small molecule vascular disrupting agents: Potential new drugs for cancer treatment. Recent Pat Anticancer Drug Discov. 2007; 2: 79-101.
- Shi LM, Fan Y, Lee JK, Waltham M, Andrews DT, Scherf U, et al. Mining and visualizing large anticancer drug discovery databases. J Chem Inf Comput Sci. 2000; 40: 367-379.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Natl Cancer Inst. 1991; 83: 757-766.
- Sakr HM, Ayyad RR, Mahmoud K, Mansour AM, Ahmed AG. Design Synthesis of Analgesics and Anticancer of Some New Derivatives of-Benzimidazole. International Journal of Organic Chemistry. 2021; 11: 144-169.
- Mohamed M Khalifa, Helmy M Sakr, Albaraa Ibrahim, Ahmed M Mansour, Rezk R Ayyad. Design and synthesis of new benzylidene-quinazolinone hybrids as potential anti-diabetic agents: In vitro a-glucosidase inhibition, and docking studies. Journal of Molecular Structure. 2022; 1250: 131725.
- Abdel-Ghany El-Helby, Helmy Sakr, Rezk Ayyad and Hazem Mahdi, et al. Design, synthesis, molecular modeling, in vivo studies and anticancer activity evaluation of new phthalazine derivatives as potential DNA intercalators and topoisomerase II inhibitors.” Bioorganic Chemistry. 2020; 103: 104233.
- Rezk Ayyad. “Synthesis and biological evaluation of novel iodophthalazinedione derivatives as anticonvulsant agents.” Al-Azhar J Pharm Sci. 2012; 45: 1-13.
- Wagdy M Eldehna, Sahar Abou-Seri, Ahmed ElKerdawy, Rezk R, et al. “Increasing the binding affinity of VEGFR-2 inhibitors by extending their hydrophobic interaction with the active site: Design, synthesis and biological evaluation of 1-substituted-4-(4-methoxybenzyl) phthalazine derivatives.” Eur J Med Chem. 2016; 113: 50-62.
- El-Helby Abdel Ghany A, Rezk R Ayyad, Helmy M Sakr, Adel S Abdulrahim, et al. “Design, synthesis, molecular modeling and biological evaluation of novel dihydrophthalazine-1, 4-dione derivatives as potential anticonvulsant agents.” J Mol Struct. 2017; 1130: 333-351.
- El-Helby Abdel-Ghany A, Rezk RA Ayyad, Helmy Sakr, Khaled El-Adl, Mamdouh M Ali, et al. Design, Synthesis, Molecular Docking, and Anticancer Activity of Phthalazine Derivatives as VEGFR-2 Inhibitors. Archiv der Pharmazie. 2017; 350: 1700240.
- El-Helby Abdel-Ghany A, Rezk RA Ayyad, Khaled El-Adl, Hazem Elkady. ”Phthalazine-1, 4-dione derivatives as non-competitive AMPA receptor antagonists: design, synthesis, anticonvulsant evaluation, ADMET profile and molecular docking.” Molecular Diversity. 2019; 23: 283-298.
- Eissa Ibrahim H, Ahmed M Metwally, Amany Belal, Ahmed BM Mehany. “Discovery and antiproliferative evaluation of new quinoxalines as potential DNA intercalators and topoisomerase II inhibitors.” Archiv der Pharmazie. 2019; 352: e1900123.
- Ibrahim Mohamed-Kamal, Ashraf A Abd-Elrahman, Rezk RA Ayyad, Khaled El-Adl, et al. “Design and synthesis of some novel 2-(3-methyl-2- oxoquinoxalin-1(2H)-yl)-N-(4-(substituted)phenyl) acetamide derivatives for biological evaluation as anticonvulsant agents.” Bulletin of Faculty of Pharmacy, Cairo University. 2013; 51: 101-111.
- Elhelby Abdelghany Aly, Rezk Rezk Ayyad, Mohamed Fathallah Zayed. “Synthesis and biological evaluation of some novel quinoxaline derivatives as anticonvulsant agents.” Arzneimittelforschung. 2011; 61: 379-381.
- El-Helby Abdel-Ghany A, Rezk RA Ayyad, Khaled El-Adl, Alaa Elwan. “Quinoxalin-2 (1H)-one derived AMPA-receptor antagonists: design, synthesis, molecular docking and anticonvulsant activity.” Med Chem Res. 2017; 26: 2967-2984.
- El-Helby Abdel-Ghany A, Rezk RA Ayyad, Mohamed F Zayed, Hamada S Abuelkheer, et al. “Design, synthesis, in silico ADMET profile and GABA-A docking of novel phthalazines as potent anticonvulsants.” Archiv Der Pharmazie. 2019; 352: 1800387.
- El-Helby Abdel-Ghany A, Helmy Sakr, Rezk RA Ayyad, Khaled El-Adl, et al. “Design, Synthesis, In vitro Anti-cancer Activity, ADMET Profile and Molecular Docking of Novel Triazolo [3,4-a] phthalazine Derivatives Targeting VEGFR-2 Enzyme.” Anti-Cancer Agent Med. 2018; 18: 1184-1196.
- Mohd Nassar Ekhlass, Fathy M Abdelrazek, Rezk R Ayyad, Ahmed F El- Farargy. “Synthesis and some reactions of 1-aryl-4-acetyl-5-methyl-1, 2, triazole derivatives with anticonvulsant activity.” Mini-Rev Med Chem. 2016; 16: 926-936.
- Ayyad Rezk. “Synthesis and anticonvulsant activity of 6-iodo phthalazinedione derivatives.” Al-Azhar J Pharm Sci. 2014; 50: 43-54.
- El-Helby Abdel-Ghany A, MK Ibrahim, AA Abdel-Rahman, RRA Ayyad, MA Menshawy, K El-Adl. “Synthesis, molecular modeling and anticonvulsant activity of benzoxazole derivatives.” Al-Azhar J Pharm Sci. 2009; 40: 252- 270.
- Rezk RA Ayyad, H Sakr, K El-Gamal. Synthesis, modeling and anticonvulsant activity of some phthalazinedione. Am J Org Chem. 2016; 6: 29-38.
- Rezk RA Ayyad. Synthesis and anticonvulsant activity of tetrabromophthalazinedione. AZ J Pharm Sci. 2008: 33-45.
- El-Helby Abdel-Ghany A, MK Ibrahim, MA Amin, Rezk RA Ayyad. Synthesis and anticonvulsant activity of phthalazindione derivatives. AZ J Pharm Sci. 2020.
- Ibrahim MK, AA Abdel-Rahman, RRA Ayyad, K El-Adl, F Elsherbeny, M Rashed. “Design and synthesis of some novel N-phthalimide derivatives with potential anticonvulsant activity.” Al-Azhar J Pharm Sci. 2010; 42: 305-322.
- Alaa A-M, Laila A Abou-Zeid, Kamal Eldin H El-Tahir, Rezk R Ayyad, A-A Magda, Adel S El-Azab. “Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4 (3H)-quinazolinones.” Eur J Med Chem. 2016; 121: 410-421.
- Mohamed Menshawy A, Rezk R Ayyad, Taghreed Z Shawer, A-M Alaa, et al. “Synthesis and antitumor evaluation of trimethoxyanilides based on 4 (3H)- quinazolinone scaffolds.” Eur J Med Chem. 2016; 112: 106-113.
- Hall JH, Hall ES, Wong OT. Cumulated Index Medicus. Anticancer Drug. 1992; 53: 55-62.
- Soje-Echaque E, Lim RKS. Inflammation and Anti-inflammatories. Spectrum, New York, USA. J Pharmacol Exp Ther. 1962: 138-224.
- Synthesis and anticonvulsant activity of phthalazindione derivatives. AZ. j. pharm. Sci. 2001; 28.
- Synthesis and anticonvulsant activity of phthalazindione derivatives. AZ. j. pharm. Sci. 2002; 29.
- Synthesis and anticonvulsant activity of tetrabromophthalazin-1,4-dione derivatives. AZ. j. pharm. Sci. 2008; 37.
- Modeling, Synthesis, and antihypeglycemic activity of novel quinazolinones containing sulfonylurea moiety. J. Biol. Sci. 2009: 7.
- Thesis, molecular modeling and anticonvulsant activity of benzoxazole derivatives. AZ. j. pharm. Sci. 2009; 40.
- Synthesis, Molecular Modeling and anticonvulsant activity of Benzoxazole derivatives. AZ. J pharma. Sci. 2009; 40: 252-271.
- Modeling, Synthesis, and anticonvulsant activity of some new quinazolinones. AZ. j. pharm. Sci. 2010; 41.
- Bailleux V. Design and synthesis of some novel n-phthalimide derivatives with potential anticonvulsant activity. AZ. j. pharm. Sci. 2010; 42: 305-322.
- Rezk R. Ayyad, et al. Synthesis and Biological Evaluation of some novel quinoxaline derivatives as anticonvulsant Agents. Arzneimittelforschung. 2011; 61: 379-381.
- Rezk R. Ayyad, et al. Synthesis and Biological Evaluation of novel iodophthalazinedione derivatives as anticonvulsant Agents. AZ. j. pharm. Sci. 2012; 45: 1-13.
- Design and synthesis of some novel 2-(3-mathyl-2-oxoquinoxelin-(2H)– yl)–N-(4-(substituted)phenyl) acetamide derivatives for biological evaluation as anticonvulsant Agents. Bulletin of faculty of pharmacy. Cairo University. 2013; 51: 101.
- Rezk R. Ayyad, Ahmed M. Monsour. Synthesis and anticonvulsant activit1y of 6- iodophthalazinedione derivatives. 2014; 50.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990; 82: 1107-1112.
- Allam RM, Al-Abd AM, Khedr A, Sharaf OA, et al. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicology Letters. 2018; 291: 77-85.