
Research Article
Austin J Anal Pharm Chem. 2022; 9(2): 1146.
Investigation on Chloramphenicol Residues in Poultry Meat in Bangladesh Using a Validated Liquid Chromatography-Tandem Mass Spectrometry
Hosain MZ*, Islam SMS and Kamal MM
Quality Control Laboratory, Department of Livestock Services, Savar, Dhaka, Bangladesh
*Corresponding author: Hosain MZ, Quality Control Laboratory, Department of Livestock Services, Savar, Dhaka-1343, Bangladesh
Received: June 13, 2022; Accepted: July 01, 2022; Published: July 08, 2022
Abstract
Chloramphenicol (CAP) is a broad-spectrum antibiotic widely applied in veterinary practices. Continual use of CAP in livestock production may lead to antibiotic resistance and health-related hazards. In this study, a rapid and highly sensitive analytical method based on ultra-performance liquid chromatographytandem mass spectrometry (UPLC–MS/MS) was developed and validated to detect and quantify there is due of CAP in poultry meat regarding the safety of humans and animals. Total 80 samples of poultry meat were collected from different poultry farms, and poultry meat sellers in three upazilas namely- Sadar, Mirzapur and Ghatail of Tangail district in the Dhaka division. The test analysis was performed using matrix-matched liquid chromatography-mass spectrophotometry. The chromatographic separation of the CA Presidue was carried out at 40°C temperature on a reverse-phase C18 column using a binary gradient pump, and quantification was performed by LC-MS/MS in electrospray mode. Mobile phase constituents were solvent (a) deionized water, and (b) acetonitrile. The flow rate was 0.35 mL/min and the total run time was 5 min. The method was validated in terms of selectivity, linearity, recovery, and precision following the 2021/808/EC guidelines and acceptance criteria were met in all the cases. The relative standard deviation (RSD) for precision was <11%. The linearity of the calibration curves was excellent (R2 >0.999) at concentrations of 0.25, 0.50, 0.75, 1.0, 2.0, and 5.0 μg/kg for matrix-matched CAP standard, and the range of linearity of this method was 0.0-5.0 μg/kg with R2 value greater than 0.99. The decision limit (CC-a), and detection capability (CCβ) were 0.29 μg/ kg, and 0.31 μg/kg respectively, and the recovery percentages ranged from 94 to 100 %. In this study, the levels of CAP residue in tested poultry meat samples were found below the detection limit. The overall parameters of the proposed method met the validation criteria, and the method proved to be suitable for CAP residues determination in poultry meat samples. Thus, this method could be a precise and highly desirable analytical procedure for rapid and routine analysis of CA Presidues in poultry meat, and obtained tested results in this study could be an authentic data to ensure the chloramphenicol free safety poultry meat for human consumption.
Keywords: Chloramphenicol Residues; Poultry Meat; LC-MS/MS; Method Development & Validation
Introduction
Antibiotics are widely used for therapeutic and prophylactic purposes in poultry production to promote growth and increase feed efficiencies [1]. However, the abused use of antibiotics and their presence in the food of animal origin are of serious concern due to the development of antibiotic resistance against the target pathogens, allergic reactions, and carcinogenic or teratogenic effects [2]. There is a wide range of chemical substances with antimicrobial activity used in poultry farms. Among them, chloramphenicol (CAP) is a broad-spectrum antibiotic that is frequently used in the poultry sector to enhance production due to its excellent antibacterial and pharmacokinetic properties and low price [3]. However, in humans, CAP residue leads to hematotoxic side effects, in particular chloramphenicol-induced a plastic anemia, allergic reactions, and gastrointestinal disorder [4]. For this reason, it has led to a prohibition of chloramphenicol for the treatment of animals used for food production. So, the use of the CAP is illegal for the administration of food-producing animals, and many countries including Bangladesh banned the use of chloramphenicol for the treatment of animals used for food production [5].
However, even though it is prohibited, CAP is still used in the poultry sectorbecause of its efficacy and relatively low cost as well as the availability and prevention of some infectious diseases in birds, and aquaculture [6]. In addition to its illegal use, products of animal origin can contain CAP residues because of their occurrence in the environment. According to some studies, CAP can still be found in several food matrices, suggesting its continued use [7-9]. Besides, there is little information available regarding the occurrence of its analogues in poultry meats and foods of animal origin as well as the environment.
Globally, the consumers are concerned about the safety and quality of the food they eat. For these reasons, increasing attention is paid to the risk of drug residue occurrence in foods and foods of animal origin. So, many sensitive and more specific methods were optimized and validated for the qualitative and quantitative determination of different antibiotics and their residues in food products with different analytical procedures [10-14]. Sample preparation is critical to the validity of trace analysis of antibiotic residues. Previous studies have set forth various types of pretreatment methods for CAP residues in food before chromatographic determination, including liquid-liquid extraction, solid-phase extraction, or the QuEChERS technique [14- 15]. Conventional methods for extraction of organic analytes from food samples usually consist of a homogenization step, followed by tedious liquid-liquid extraction procedures with one or more several clean-up steps and purification of the extract to remove co-extract ants, before the sample is subjected to chromatographic separation [16].
However, there are many methods for screening and quantification of CAP in food of animal origin, but sensitive and rapid methods for analyzing CAP residues in poultry meats are still very few. Therefore, the present study aimed to develop and validate rapid and precise analytical method for the detection and quantification of chloramphenicol and its residues in poultry meat using a liquid chromatography-tandem mass spectrometry.
Materials and Methods
Chemicals and Reagents
Acetonitrile (MS grade), and ethyl acetate employed in this study were purchased from Honeywell, Germany, and authorized reference standards of Chloramphenicol and Chloramphenicol D5 (Internal standard) were purchased from LGC Labor GmbH (Augsburg, Germany). Double deionized (DI) water utilized in this study was obtained from a water deionization plant (ePure-D4642-33, Thermo Fisher Scientific, USA). All solutions were sonicated and filtered through a 0.22 μm filter employing a vacuum filtration unit (Welch, Pall Scientific, USA) before use.
Instrumentation and Chromatographic & MS/MS Conditions
The liquid chromatographic-mass spectrometry system is equipped LC (UPLC- I Class) pump with binary gradient mode, and an MS detector (Xevo TQS-Micro, and Nitrogen NM32LA, Waters Corporation USA; Peak Scientific) with MassLynx data processing software. Chromatographic separation of CAP was carried out using a C18 reversed-phase LC column (Acquity UPLC BEH C18 1.7μm, 2.1x 100 mm) operating at column oven temperature of 40°C. Deionized water and acetonitrile were used as a mobile phase operated in an isocratic elution condition. The flow rate of the mobile phase was 0.35 ml/min, and the injection volume was 10 μl for standard and samples. The mass spectrometry analysis mode was a negative scan for identification with the following conditions: temperature of source and desolvation was 1500C, and 6000C respectively, and gas flow of Cone and desolvation was 50L/hr, and 1000 L/hr respectively. ES Negative multiple reaction monitoring (MRM) of 321.2 >152.2 for quantification of CAP, and MRM of 326.2>157 for quantification of Internal Standard (CAP-D5) were used at retention time 5.0 min.
Sample Collection
Poultry meat samples (n = 80) samples were collected from different poultry farms and meat sellers in three Upazilas of Tangail district in Dhaka division. Each sample (10 g) was homogenized using a kitchen blender and was taken in a screw cap Teflon tube (50 ml) and stored at -20°C until analysis.
Preparation of Standard Solution
Stock standard solution of 1000 μg/mL was prepared by weighing 10 mg of the Chloramphenicol and Internal standard (CAP-D5) in a 10 mL amber color volumetric flask separately and diluted to volume with MS grade acetonitrile. These solutions were used as reference stock standard solutions and kept in a refrigerator at -200C for further use. Intermediate standard solutions of 100 μg/mL of CAP and CAP-D5 were prepared from stock standard solution in acetonitrile. Working standard solutions were prepared daily from intermediate standard solutions. Before injecting into the liquid chromatography system, the standard solutions were filtered through a 0.20 μm polyvinylidene fluoride (PVDF) syringe filter.
Preparation of Sample Solution
Weighed portions (poultry meat: 2 ± 0.01 g) of blended sample in 50 ml screw-capped plastic falcon tube. Spiked standard and working internal standard solution to all tubes. Vortex for 5 min and wait for 15 minutes. Added 10 ml ethyl acetate and vortex for 10 minutes. The solution was centrifuged at 6500 rpm for 10 minutes at 10°C temperature. Collected the upper layer (ethyl acetate-5 ml) and transferred it to the 15 ml screw-capped tube, and repeated the same procedure a second time. Then, evaporated the solvent (ethyl acetate) under N2 gas at 40°C temperature. Reconstituted the remaining portion attached to the bottom with 2 ml of 50% ACN. The solution was vortex for 3 minutes and centrifuged for 5 minutes at 10°C temperature. Finally, collected the supernatant and filtered with a 0.22 μm PVDF filter and transferred to the sample vial for analysis with LC-MS/MS.
Method Validation Parameters
Method validation of the present study was performed by measuring the essential parameters of the validation process like specificity, linearity and calibration curve, recovery, precision, and decision limit. The validation parameters were evaluated in accordance with 2021/808/EC guidelines [17].
Linearity and Calibration Curve: To determine the linear range and calibration curve six spiked poultry meat samples (starting from 0.25 to 5.0 μg/kg) have been prepared. Then run the spiked matrixmatched standard solution. The matrix-matched standard calibration curve was prepared with all data and was linear in the concentration range of 0.0-5.0 μg/kg.
Selectivity: Demonstration of the absence of interference from the ingredients in the Poultry meat sample by LC-MS/MS. The selectivity of the test method in this study was evaluated by measuring the peak area of reagent blank solution, matrix blank solution, standard solution, and spiked sample solution.
Recovery: Three sets of spike samples at 1.0, 2.0, and 3.0 times the LCL (lowest calibrated level) level have been prepared and analyzed; each level of each set contained six replicate samples. The LCL of the linearity curve was 0.25 μg/kg. Therefore, three sets of spike samples were 0.25 μg/kg, 0.50 μg/kg, and 0.75 μg/kg. Raw data were calculated using the following equation.
Recovery (%) = (measured content / fortification level) X100.
Repeatability Precision: For repeatability precision check three sets of samples have been prepared to spike at 1.0, 2.0, and 3.0 times the LCL level, and analyzed as before. The mean concentration, standard deviation, and the coefficient of variation (%) of each level of fortified samples have been calculated. Finally, the overall mean concentrations and CVs for the fortified samples have been calculated.
Within-laboratory Reproducibility: For within-laboratory reproducibility precision check, three sets of samples have been prepared to spike at 1.0, 2.0, and 3.0 times the LCL level, and analyzed by the second analyst as before. The mean concentration, standard deviation, and the coefficient of variation (%) of each level of fortified samples have been calculated.
Matrix Effect: Matrix effect (%) was calculated with reference to the peak area of standard spiked control matrix and peak area of standard solution in a solvent. The matrix effect was evaluated by using the matrix-matched calibration.
Decision Limit (CCa): To determine the decision limit (CCa), 20 blank poultry meat samples have been fortified with chloramphenicol at the LCL level (0.25 μg/kg) and analyzed. The decision limit (CCa) was calculated using the following equation
CCa = CLCL + 2.33xSD20representative samples spiked at LCL level
Detection Capability (CCβ): To determine the detection capability (CCβ), 20 blank poultry meat samples have been fortified with the chloramphenicol at the LCL level (0.25 μg/kg) and analyzed. The detection capability (CCβ), was calculated using the following equation
CCβ = CCa + 1.64 xSD20 representative samples spiked at LCL level
Analysis of the Real Sample
The validated LC-MS/MS method was used to analyze collected thirty poultry meat and thirty beef samples (every three replicates). The levels of CAP in tested samples were found below the detection limit.
Statistical Analysis
The data obtained in this study were analyzed with the Masslynx software and Statistical Package for the Social Sciences version 16 (SPSS-16) statistical package by one-way analysis of variance, and in regression analysis, the least square method was performed.
Results and Discussion
This study reveals the development and validation of a definite analytical method where the validation criteria [17] are met in all the cases. Typical chromatograms of the matrix-matched solution spiked with standard and internal standards are shown in (Figure 1). The retention time of both CAP and internal standard (CAP-D5) was 1.77±0.01 min. The selectivity test results (Figure 1 and Table 1) of the assay method demonstrate the absence of interference with the elution of CAP, and CAP-D5 in the matrix blank sample. From Figure 2, demonstrates the excellent linearity (R²>0.999) within the concentration range of 0.25-5.0 μg/kg. The range of linearity of this method was 0.0-5.0 μg/kg with an R² value greater than 0.999. The trueness of the method was determined by recovery percentage and the values are between 101 % and 105% (Table 2), which suggests that the method is accurate and also indicates that the commonly used excipients present in the poultry meat are not interfere with the proposed method. The precision for the method and analyst was evaluated which are shown in (Tables 3a and b). The results demonstrate that the RSD value for both cases is <10%, which indicates that the proposed method has excellent reproducibility. The matrix enhancement effects were86.67 % which indicates that the sample matrix interfered with the detection of CAP residues. So, matrix-matched calibration curves were used for quantitative analysis of CAP. The decision limit (CCa), and detection capability (CCβ) for CAP are 0.29μg/kg, and0.31 μg/kg respectively.
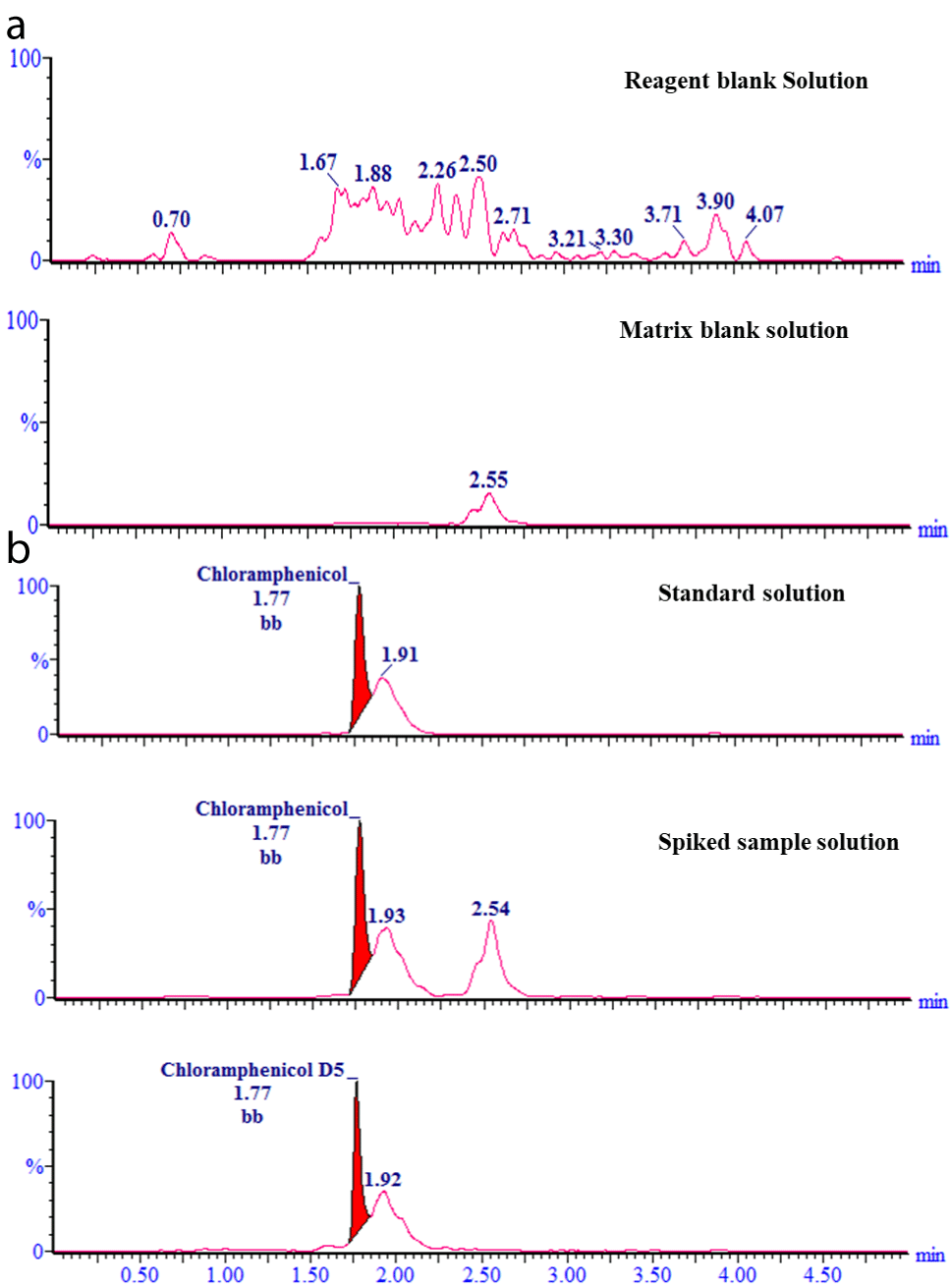
Figure 1: Typical chromatograms of the matrix-matched solution spiked with
standard and internal standard.
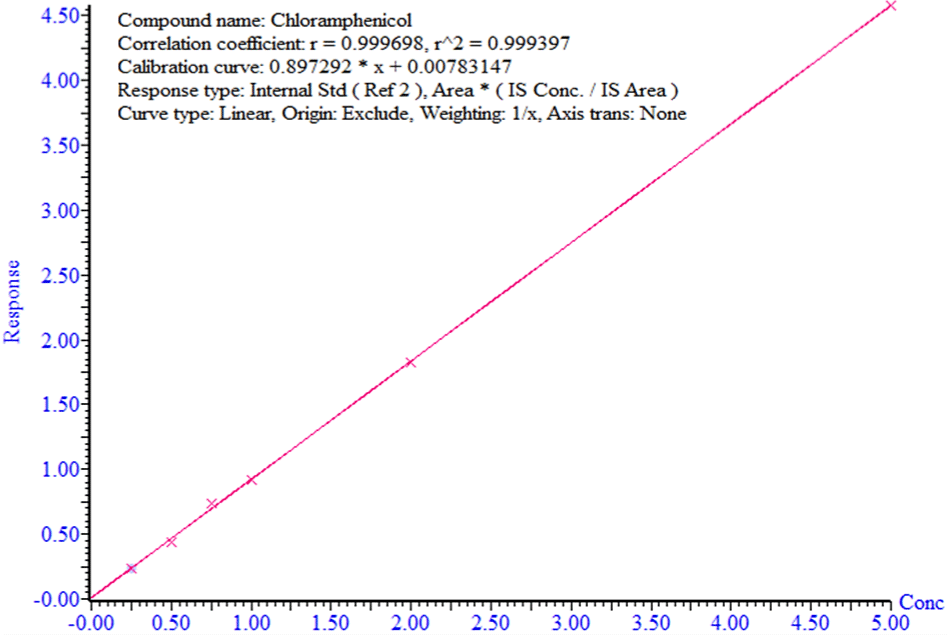
Figure 2: Matrix-matched calibration curve of chloramphenicol.
Sample Name
Retention Time (min)
Response/Peak area
Reagent blank Solution
Nil
Nil
Matrix blank solution
Nil
Nil
Standard solution (0.25 μg/kg)
1.77
483
Spiked sample solution (0.25 μg/kg)
1.77
558
Table 1: Selectivity test results.
Replicate
Recovery (%)
0.25 μg/kg
0.50 μg/kg
0.75 μg/kg
1
97.8
102.9
103.4
2
106.7
99.3
98.8
3
102.6
102.5
106
4
95.7
96.4
110.3
5
105.5
105.9
106.3
6
105.4
99
107
Average
102.28
101
105.3
Table 2: Recovery of the method for the chloramphenicol in poultry meat.
Replicate
Conc(μg/Kg)
Conc(μg/Kg)
Conc(μg/Kg)
Inj-01
0.25
0.51
0.78
Inj-02
0.27
0.50
0.74
Inj-03
0.26
0.51
0.8
Inj-04
0.24
0.48
0.83
Inj-05
0.26
0.53
0.80
Inj-06
0.26
0.50
0.80
Average
0.26
0.50
0.79
SD
0.01
0.02
0.03
% RSD
4.49
3.33
3.69
Table 3a: Precision under repeatability conditions (n=6).
Injection
First analyst
Second analyst
% RSD (0.25 μg/kg)
% RSD (0.50 μg/kg)
% RSD (0.75 μg/kg)
% RSD (0.25 μg/kg)
% RSD (0.50 μg/kg)
% RSD (0.75 μg/kg)
Inj-01
0.25
0.51
0.78
0.27
0.46
0.68
Inj-02
0.27
0.50
0.74
0.22
0.44
0.69
Inj-03
0.26
0.51
0.80
0.24
0.45
0.72
Inj-04
0.24
0.48
0.83
0.27
0.46
0.71
Inj-05
0.26
0.53
0.80
0.29
0.45
0.76
Inj-06
0.26
0.50
0.80
0.24
0.44
0.76
Average
0.26
0.50
0.79
0.25
0.45
0.72
SD
0.01
0.02
0.03
0.02
0.01
0.03
% RSD
4.49
3.33
3.69
9.46
1.45
4.87
Table 3b: Within-laboratory reproducibility (n=6).
Some LC-MS/MS methods have been published concerning the simultaneous determination of CAP in food and feed samples [18-22] with the different chromatographic conditions, longer retention time, and poor recoveries [23]. Although, these methods were reported for quantification of CAP in different sample matrices such as feed, meat, milk, honey, and some biological matrices [24-26] they are incompetent to analyze poultry meat. The method we developed and validated is more precise with good selectivity, linearity, precision, and high recovery that met all the criteria of the validation parameters (Table 4). Moreover, the application of the method to test samples showed no false negative or false positive results even after the analysis of a significant number of samples. In our study, total of 80poultry meat samples were tested for analysis of chloramphenicol and there was no positive sample found in the study (Table-5). Although the samples were collected from the selected area in Bangladesh, the real status of the use of banned antibiotic chloramphenicol in poultry farms was figured out in this study.
Parameters
Acceptance criteria
Obtained results
Selectivity
The excipient compounds must not interfere with the analysis of the targeted analyte.
Chromatography shows-
(i) the existence of peak area in standard solutions and real sample;
(ii) absence of peak area in blank and matrix solutions.Linearity
R2> 0.99
R2> 0.999
Trueness by Recovery
Recovery should be between 50 to120 %
101 to 105 %.
Precision (Repeatability/ Within-laboratory reproducibility precision)
Repeatability: RSD <20 %
Within-laboratory reproducibility : RSD <30 %Repeatability: 3.33 to 4.49 %
Reproducibility precision: 1.45 to 9.46 %Matrix effect (%)
-
86.67%
Decision Limit (CCa)
CCa = CLCL +2.33xSD20
0.29 μg/Kg
Detection capability (CCβ)
CCβ = CCa + 1.64 x SD20 representative samples spiked at LCL level
0.31 μg/Kg
Table 4: Summary of acceptance criteria and obtained results.
Name of Upazila
Type of chicken meat sample
Number of meat sample
Total no. of test
Results
Broiler
Layer
Sonali
Cockerel
Tangail Sadar
12
3
3
2
20
20
below detection level
Mirzapur
15
2
5
3
25
25
below detection level
Ghatail
18
4
10
3
35
35
below detection level
Table 5: Test results summary of chloramphenicol residues in poultry meat samples (n=80).
The easy sample extraction procedure and short run time of less than five minutes make the procedure more convenient. Thus, the proposed method could be a simple, precise, and rapid analytical technique for simultaneous detection and quantification of CAP in poultry meat at the trace level.
Conclusion
In this study, a rapid and precise method has been developed and validated with good linearity, precision, and high accuracy for detection and quantification of chloramphenicol in poultry meat samples that meets all the criteria mentioned in the 2021/808/EC guidelines. The mobile phase preparation and sample extraction procedure in the proposed method are simple and quantification of chloramphenicol in the real samples is also comparable with excellent recovery. Values of CCa and CCβ obtained for the chloramphenicol in poultry meat are very close to the minimum required performance limit (MRPL) value which indicates its reliability. Thus, the proposed method might be a specific and sensitive method for quantification of banned antibiotic chloramphenicol and its residue in poultry meat samples, and the obtained test results could be a reference data or a strong poof regarding the chloramphenicol free poultry meat concerning the food safety issue in Bangladesh, and it will be helpful to create an opportunity for exporting the poultry meat in abroad.
Acknowledgments
The work was supported by the “Quality Control Laboratory for Livestock Inputs and its Food Products (QC Lab)” establishment project (No. 224071600) under the Department of Livestock Services, Government of Bangladesh. We are grateful to Analytical Division, Techno worth Associates Ltd., Bangladesh, for their technical support.
References
- Roth N, Käsbohrer A, Mayrhofer S, Zitz U, Hofacre C, Domig KJ. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poultry Science. 2019; 98(4): 1791-1804. doi:10.3382/ps/pey539.
- Okocha RC, Olatoye IO, Adedeji OB. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Reviews. 2018; 39(1). doi:10.1186/s40985-018-0099-2.
- Jung H, Park D, Choi Y, Kang S, Cho H, Choi J, et al. Simultaneous Quantification of Chloramphenicol, Thiamphenicol, Florfenicol, and Florfenicol Amine in Animal and Aquaculture Products Using Liquid Chromatography- Tandem Mass Spectrometry. Frontiers in Nutrition. 2021; 8. doi:10.3389/ fnut.2021.812803.
- Mou SA, Islam R, Shoeb M, Nahar N. Determination of chloramphenicol in meat samples using liquid chromatography–tandem mass spectrometry. Food Science & Nutrition. 2021; 9(10): 5670-5675. doi:10.1002/fsn3.2530.
- Imran M, Fazal-e-Habib, Tawab A, Rauf W, Rahman M, Khan QM, et al. LC-MS/MS based method development for the analysis of florfenicol and its application to estimate relative distribution in various tissues of broiler chicken. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2017; 1063: 163-173. doi:10.1016/j.jchromb.2017.08.029.
- Xie X, Wang B, Pang M, Zhao X, Xie K, Zhang Y, et al. Quantitative analysis of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in eggs via liquid chromatography-electrospray ionization tandem mass spectrometry. Food chemistry. 2018;.269:.542-548. doi:10.1016/j.foodchem.2018.07.045.
- Samsonova JV, Cannavan A, Elliott CT. A critical review of screening methods for the detection of chloramphenicol, thiamphenicol, and florfenicol residues in foodstuffs. Crit Rev Anal Chem. 2012; 42(1): 50-78.
- Wang B, Xie K, Lee K. Veterinary Drug Residues in Animal-Derived Foods: Sample Preparation and Analytical Methods. Foods. 2021; 10(3): 555. doi:10.3390/foods10030555.
- Martins Júnior HA, Bustillos OV, Pires MA, Lebre DT, Wang AY. Determination of chloramphenicol residues in industrialized milk and honey samples using LC-MS/MS. Química Nova. 2006; 29(3): 586-592.
- Bogusz MJ, Hassan H, Al-Enazi E, Ibrahim Z, Al-Tufail M. Rapid determination of chloramphenicol and its glucuronide in food products by liquid chromatography-electrospray negative ionization tandem mass spectrometry. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2004; 807(2): 343-356. doi:10.1016/J. JCHROMB.2004.04.027.
- Ramatla T, Ngoma L, Adetunji M, Mwanza M. Evaluation of Antibiotic Residues in Raw Meat Using Different Analytical Methods. Antibiotics. 2017; 6(4): 34. doi:10.3390/antibiotics6040034.
- Zhang S, Liu Z, Guo X, Cheng L, Wang Z, Shen J. Simultaneous determination and confirmation of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in chicken muscle by liquid chromatography-tandem mass spectrometry. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2008; 875(2): 399-404. doi:10.1016/j. jchromb.2008.09.035.
- Sniegocki T, Sell B, Giergiel M, Posyniak A. QuEChERS and HPLC-MS/MS Combination for the Determination of Chloramphenicol in Twenty Two Different Matrices. Molecules. 2019; 24(3): 384. doi:10.3390/molecules24030384.
- Rønning HT, Einarsen K, Asp TN. Determination of chloramphenicol residues in meat, seafood, egg, honey, milk, plasma and urine with liquid chromatography-tandem mass spectrometry, and the validation of the method based on 2002/657/EC. Journal of chromatography. A. 2006; 1118(2): 226- 233. doi:10.1016/J.CHROMA.2006.03.099.
- Pfenning AP, Roybal JE, Rupp HS, Turnipseed SB, Gonzales SA, Hurlbut JA. Simultaneous determination of residues of chloramphenicol, florfenicol, florfenicol amine, and thiamphenicol in shrimp tissue by gas chromatography with electron capture detection. Journal of AOAC International. 2000; 83(1): 26-30. doi:10.1093/JAOAC/83.1.26.
- Wu X, Shen X, Cao X, Nie R, Zhang H, Tang C, et al. Simultaneous Determination of Amphenicols and Metabolites in Animal-Derived Foods Using Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry. International Journal of Analytical Chemistry. 2021; 2021: 1-10. doi:10.1155/2021/3613670.
- 2021/808/EC. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing Decisions 2002/657/EC and 98/179/EC. Offical Journal of the European Union. 2021; L180: 84–109.
- Tölgyesi Á, Fekete J, Sharma VK, Palffi É, Bekesi K, Lukonics D, Pleva G. A LC-MS/MS confirmatory method for determination of chloramphenicol in real samples screened by competitive immunoassay. Acta Aliment. 2014; 43(2): 306-314.
- Patyra E, Kwiatek K. Quantification and Analysis of Trace Levels of Phenicols in Feed by Liquid Chromatography–Mass Spectrometry. Chromatogr. 2020; 83(6): 715-723.
- Xie K, Jia L, Yao Y, Xu D, Chen S, Xie X, et al. Simultaneous determination of thiamphenicol, florfenicol and florfenicol amine in eggs by reversed-phase high-performance liquid chromatography with fluorescence detection. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2011; 879(23): 2351-2354. doi:10.1016/j.jchromb.2011.06.027.
- Pietro WJ, Wozniak A, Pasik K, Cybulski W, Krasucka D. Amphenicols stability in medicated feed – development and validation of liquid chromatography method. Bulletin of the Veterinary Institute in Pulawy. 2014; 58(4): 621-629. doi:10.2478/BVIP-2014-0095.
- Patyra E, Kwiatek K. Determination of fluoroquinolones in animal feed by ion pair high-performance liquid chromatography with fluorescence detection. Anal Lett. 2017; 50(11): 1711-1720.
- Mishra A, Chhonker YS, Bisen AC, Prasad YD, Tulsankar SL, Chandasana H, et al. Rapid and Simultaneous Analysis of Multiple Classes of Antimicrobial Drugs by Liquid Chromatography-Tandem Mass Spectrometry and Its Application to Routine Biomedical, Food, and Soil Analyses. ACS Omega. 2020; 5(49): 31584-31597. doi:10.1021/acsomega.0c03863.
- Guidi LR, Silva LH, Fernandes C, Engeseth NJ, Gloria MB. LC-MS/MS determination of chloramphenicol in food of animal origin in Brazil. Scientia Chromatographica. 2015; 7(4): 287-295.
- Jung H, Park D, Choi Y, Kang S, Cho H, Choi J, et al. Simultaneous Quantification of Chloramphenicol, Thiamphenicol, Florfenicol, and Florfenicol Amine in Animal and Aquaculture Products Using Liquid Chromatography- Tandem Mass Spectrometry. Frontiers in Nutrition. 2021; 8. doi:10.3389/ fnut.2021.812803.
- Sniegocki T, Gbylik-Sikorska M, Posyniak A. Analytical Strategy for Determination of Chloramphenicol in Different Biological Matrices by Liquid Chromatography - Mass Spectrometry. Journal of Veterinary Research. 2017; 61(3): 321-327. doi:10.1515/jvetres-2017-0032.