
Research Article
Austin J Anal Pharm Chem. 2022; 9(3): 1149.
Ulcer Index, Analgesics and Anti inflammatory Screening of Some Arylidene Compounds Derived from Phenyl Hydrazine and Aromatic Aldehydes
Salem M¹*, Ayyad R², Sakr H², El-Helby AE², Mansour A³ and Mahmoud K¹
1Medicinal Chemistry Department, Faculty of Pharmacy, Sinai University, Egypt
2Pharmaceutical Chemistry Department, Faculty of Pharmacy, Al-Azhar University, Egypt
3Pharmacology department, Faculty of Pharmacy, Al-Azhar University, Egypt
4Pharmaceutical Chemistry department, Faculty of Pharmacy, Egyptian Russian University, Egypt
*Corresponding author: Mo’men Salem, Medicinal Chemistry Department, Faculty of Pharmacy, Sinai University, Northern Sinai, Egypt
Received: August 22, 2022; Accepted: October 14, 2022; Published: October 21, 2022
Abstract
Arylidene derivatives were synthesized from phenyl hydrazine and series of different aldehydes. Arylidene compounds are important classes of N-containing fused heterocycles widely used as key building blocks for pharmaceutical agents due to a wide range of biological activities that include anti-inflammatory and antitumor properties [1]. Arylidene derivatives were furnished by the fusion of benzene ring with different aldehydes via hydrazine moiety in the presence of glacial acetic acid. These derivatives were characterized by TLC, melting points, Infrared Red, Nuclear Magnetic Resonance and Mass Spectroscopy. Finally, these synthetized derivatives were tested for analgesic activity using hot plate test method, anti-inflammatory activity using rat paw oedema and ulcer index test.
Keywords: Arylidene; phenyl hydrazine; Aromatic aldehydes; Benzylidenes; Analgesic activity; Anti-inflammatory activity; Hot plate test; Rat paw oedema; Ulcer index
Introduction
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are a significant class of pharmacological agents with broad therapeutic applications in the treatment of fever, inflammation, and pain [2]. Their application in the treatment of chronic inflammatory diseases, like Rheumatoid Arthritis (RA) and Osteoarthritis (OA) for long period of time was found to have negative side effects which limits their clinical utility [2]. The reported mechanism of NSAID-associated GI adverse effects is the suppression of prostaglandin biosynthesis from arachidonic acid by nonselective inhibition of the two isoforms of Cyclooxygenase (COX) enzyme (COX-1 and COX-2) (2). The tricyclic selective COX-2 inhibitors (coxibs) like celecoxib, rofecoxib, and valdecoxib showed remarkable gastrointestinal safety profile with the same antiinflammatory efficacy as classical NSAIDs. However, concerns about the cardiovascular safety of coxibs have been raised after withdrawal of rofecoxib and valdecoxib from the market [3,4]. It was suggested that the presence of certain moieties within the chemical structure of coxibs are responsible for the increased cardiovascular risk [5]. Therefore, continuous research on the development of novel antiinflammatory agents with increased COX-2 selectivity while moving away from the classic coxib structures could provide agents with improved cardiovascular and GI safety profiles [5]. It was reported that the usage of arylidene moeities considered as a corner stone in the synthesis of many biologically active moleculess especially as antiinflammatory and anticancerous agents [6,7]. In this work, organic compounds using phenyl hydrazine and series of aromatic aldehydes are synthetized and tested for their anti-inflammatory, analgesic and ulcerogenic activity. We targeted the synthesis of compounds formed of benzene ring attached by hydrazine moiety which is two nitrogen atoms but not fused in the ring as Phthalazines, Quinazolines, Quinoxalines or Benzimidazoles [8-20]. These new compounds having two nitrogen atoms in side chain as a bridge between benzene ring and aromatic aldehydes.
Materials
Reagents
All solvents and reagents were obtained from commercial sources and were used without further purification except Glacial Acetic acid and Petroleum Ether (PE). Phenyl Hydrazine was purchased from Sigma Aldrich (Cairo, Egypt). Series of Aromatic Aldehydes were acquired from Sigma Aldrich (Cairo, Egypt). Absolute Ethanol, Ehanol 95%, Glacial Acetic Acid, Ethyl Acetate, Petroleum Ether and Chloroform were purchased from Piochem (Cairo, Egypt). Distilled water was used for the experiments.
Instruments
Progress of chemical reactions was observed using TLC (Merck, silica gel plates 60 F254) and visualized using a UV–Vis spectrometer at 254 nm. Melting points were determined by Mel-Temp apparatus. NMR spectra were performed in Chloroform (7.26 ppm), with trimethyl silane as an internal standard, using Bruker Avance 500 spectrometer at ambient temperature, at drug discovery unit, Faculty of Pharmacy, Ain Shams University (ASU, Cairo, Egypt). All chemical shifts were expressed in parts per million (d), and coupling constants (J) in Hz. FTIR spectra were recorded using KBr pellets on a model 883 double beam infrared spectrophotometer Bruker in 200– 4000 cm-1, at drug discovery unit, Faculty of Pharmacy, Ain Shams University (ASU,Cairo, Egypt). MS spectra were recorded using a Bruker Esquire 2000 by APC or ES ionization, at drug discovery unit, Faculty of Pharmacy, Ain Shams University (ASU, Cairo, Egypt).
Hot Plate Test for Analgesic Activity
Eighty-four male Swiss albino mice (22 ± 2 gm), 6-8 weeks of age were used in this study. The animals were obtained from the breeding colony maintained at the animal house of the El-Nile Pharmaceutical and Chemical Industries Company, Cairo, Egypt. Animals were kept in a controlled environment (25 ± 2°C, 50 ± 5 % humidity) under a 12-hour light/dark cycle and had free access to a standard pellet chow and tap water throughout the study. All experiments were conducted at Al-Azhar University, Faculty of Pharmacy, Cairo, Egypt. Experiments were conducted according to the recommendations of the ethics committee for animal welfare and have been approved by Institutional Animal Care and Utilization. Hot plate test was used to estimate analgesic activity by the method explained by Eddy and Leimbach [21]. Mice were retained on a hot plate having a stable temperature of 55 ± 1°C. The time taken for either paw licking or jumping was recorded. Each mouse was individually placed on the hot plate in order to find the animal’s reaction to electrical heatinduced pain (licking of the fore paws and eventually jumping). The latency until mice showed first signs of discomfort (hind paw lifting, hind paw licking, or jumping) were recorded, before (baseline), and response was determined after 60 min the administration of normal saline, indomethacin (10 mg/kg), diclofenac sodium (7 mg/ kg) (Figure 1) and the tested compounds (1-11) (Figure 2) (10mg/ kg). Data were analyzed using statistical software Graph Pad Prism version 5. One-way analysis of variance (ANOVA) test was used to ascertain the significance of variations between the times of licking in hot plate test. All data were considered significant at p< 0.05 [22].
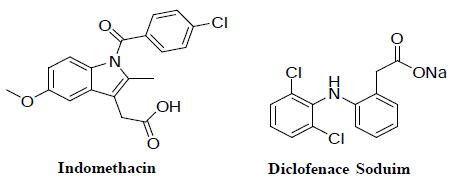
Figure 1: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
Rat Paw Oedema for Anti-Inflammatory Activity
Ninety adult healthy Male Wister albino rats weighting between 140-160 gm were used for the study. The animals were obtained from the breeding colony maintained at the animal house of the El-Nile Pharmaceutical and Chemical Industries Company, Cairo, Egypt. The animals were housed in standard conditions (temperature 25 ± 2°C with 55 ± 5% relative humidity and a 12-hour light dark cycle). All animals had free access to water and normal diet. The study was approved by Institutional Animal Ethical Committee (IAEC) and was in accordance with the guideline of the Committee for the Purpose of Control and Supervision of Experimental Animal (CPCSEA). The initial paw volume of each rat was noted by the usage of caliper. Ninety animals were used in this study and divided into 15 groups (six animals per each).
Group-1 was served for saline injection in the right hind paw.
Group-2 was served for carrageenan injection in the right hind paw.
Group-3 was served for diclofenac sodium injection at a dose of 7 mg/kg body weight.
Group-4 was received indomethacin at a dose of 10 mg/kg body weight.
Group 5-15 received the test compounds at a dose of 200 mg/kg body weight.
One-hour post oral administration of the tested compounds (Figure 1) at a dose of 200 mg/kg, standards (indomethacin (10 mg/ kg orally) and diclofenac sodium (7 mg/kg I.P) (Figure 1)), 1% w/v from carrageenan solution (0.1 ml/paw) was injected subcutaneously into the plantar surface of the rat right leg hind paw. The paw volume of the left legs; negative control for each animal was measured with the help of official digital caliper during the time intervals of 1, 2, 3, 8, 12 and 24 h after carrageenan injection.
Percentage protection (or inhibition) was calculated by using the formula,
% protection = (1-Vt/Vc) *100
Vt is the mean increase in the paw volume in the test animals group,
Vc is the mean increase in the paw volume in the control group [23].
Ulcer Index
Fresh solution of indomethacin (Figure 1) 25 mg/kg was purchased from (El-Nile Co. for Pharmaceuticals and chemical industries, Cairo, Egypt) dissolved in sterile water for injection and the tested compounds (1-11) (Figure 2) were dissolved in DMSO. Seventy-two male Sprague Dawly rats weighing between 150 ± 10 gm were used for the study. Prior to the experiments, the rats were kept in the animal house for one week for acclimatization in rat cages and were given standard rat feed with water ad libitum. The animals were kept fasting for 24 hours before carrying out the experiments.

Figure 2: Tested compounds (1-11).
Rats were divided into 12 groups of 6 each
• Group I: Control (1 ml/kg DMSO solvent orally).
• Group II: Indomethacin (25 mg/kg orally).
• Group III: Compound 1 (600 mg/kg orally in DMSO solvent).
• Group IV: Compound 2 (600 mg/kg orally in DMSO solvent).
• Group V: Compound 3 (600 mg/kg orally in DMSO solvent).
• Group VI: Compound 4 (600 mg/kg orally in DMSO solvent).
• Group VII: Compound 5 (600 mg/kg orally in DMSO solvent).
• Group VIII: Compound 6 (600 mg/kg orally in DMSO solvent).
• Group IX: Compound 7 (600 mg/kg orally in DMSO solvent).
• Group X: Compound 8 (600 mg/kg orally in DMSO solvent).
• Group XI: Compound 9 (600 mg/kg orally in DMSO solvent).
• Group XII: Compound 10 (600 mg/kg orally in DMSO solvent).
The rats were sacrificed by cervical dislocation 3 hours after indomethacin (Figure 1) and tested compounds (1-11) administration (Figure 2). The abdomen was dissected to retrieve stomach, analyzed for ulcer index. With small nick, fundus of stomach was perforated on greater curvature of stomach. After ligation of cardiac and pyloric end, the greater curvature of stomach was opened. Gastric mucosa was observed under magnifying glass to calculate the ulcer index [24]. Measurement of gastric ulcerations following their induction was achieved by dissecting the stomach along its greater curvature and fixing on a board or transparent glass. Examination can be carried out macroscopically with a hand lens and by tracing on a transparent paper after which the transparent paper is placed onto a graph sheet and sizes of ulcers are measured. According to the method by Kulkarni, the ulcer index can be measured or registered using the following scores involving the number and severity of ulcers:
0.0 = normal colored stomach 0.5 = red coloration,
1.0 = spot ulcers 1.5 = hemorrhagic streaks,
2.0 = ulcers with area > 3 but ≤ 5mm²
Ulcer index (UI)=total ulcer score/no of animals ulcerated
Chemistry and Scheme
Scheme:
Procedure and synthesis of Compounds 3-13: Equimolar mixture of Phenyl hydrazine and series of Aromatic Aldehydes were stirred together in refluxing glacial acetic acid (Figure 3). TLC was made by 2:1 Petroleum Ether: Ethyl Acetate system. Precipitate was obtained from organic layer then water was added and more precipitate was retrieved. Product was purified by crystallization in Absolute Ethanol.

Figure 3: Reagents and conditions: Series of Aromatic Aldehydes, Refluxing
Glacial Acetic Acid, 135°C, 1-16 Hrs.
Compound 1: (E)-1-benzylidene-2-phenylhydrazine: Yield 70%. m.p = 154-156 °C. IR: 688.75, 747.51 cm-1 (aromatic, bending), 880.40 cm-1 (N-H, overtone), 1064.45 cm-1 (C-N), 1518 cm-1 (N-H, bending), 1590 cm-1 (C=C, aromatic), 2450 cm-1 (aromatic, overtone), 3090 cm-1 (C-H, aromatic) and 3300 cm-1 (N-H, stretching). 1HNMR (400 MHz, CDCl3): d 6.90-7.50 ppm (m, aromatic protons), 7.65 ppm (s, -CH-) and 10.3 ppm (s, -NH-). 13CNMR (100 MHz, CDCl3): d C1 (144.5 ppm), C2 (117 ppm), C3 (114 ppm), C4 (137 ppm), C5 (114 ppm), C6 (117 ppm), C7 (146 ppm), C1 (147.5 ppm), C2 (115 ppm), C3 (130 ppm), C4 (125 ppm), C5 (130 ppm) and C6 (115 ppm).
Compound 2: (E)-1-(4-methoxybenzylidene)-2- phenylhydrazine: Yield 82.5%. m.p = 128–130 °C.¹HNMR (400 MHz, CDCl3): d 3.86 ppm (s,-CH3-), 6.85-7.35 ppm (m, aromatic protons), 7.65 ppm (s,-CH-) and 9.9 ppm (s,-NH-).13CNMR (100 MHz, CDCl3): d C1 (54.3 ppm), C2 (158.9 ppm), C3 (113.6 ppm), C4 (129.8 ppm), C5 (124.8 ppm), C6 (129.8), C7 (113.6 ppm), C8 (143.8 ppm), C1 (145.2 ppm), C2 (112.2 ppm), C3 (129.5 ppm), C4 (128.8 ppm), C5 (129.5 ppm) and C6 (112.2 ppm).
Compound 3: (E)-1-(2-chlorobenzylidene)-2-phenylhydrazine: Yield 73%. m.p = 129-131 °C.1HNMR (400 MHz, CDCl3): d 6.75- 7.75 ppm (m, aromatic protons), 7.85 ppm (s, -CH-) and 10.5 ppm(s, -NH-).MS: m/z: 230.06 (100.0%), (M+1) 231.05 (87.9%), (M+2) 229.05 (12.1%).
Compound 4: 4-((2-phenylhydrazono)methyl)phenol: Yield 86 %. m.p = 178–181 °C.IR: 690.59, 743.83 cm-1 (aromatic, bending), 884.73 cm-1 (N-H, overtone), 1098.33 cm-1 (C-N), 1504 cm-1 (N-H, bending), 1596.49 cm-1 (C=C, aromatic), 1700 cm-1 (C=N), 3045 cm-1 (C-H, aromatic), 3290 cm-1 (N-H, stretching) and 2900-3625 cm-1 (OH).¹HNMR (400 MHz, CDCl3): d 6.85-7.55 ppm (m, aromatic protons), 7.7 ppm (s, -CH-), 7.85 ppm (s, -OH) and 9.88 ppm (s, -NH-).13CNMR (100 MHz, CDCl3): d C1 (158.82 ppm), C2 (117.56 ppm), C3 (130.8 ppm), C4 (125.4 ppm), C5 (130.8 ppm), C6 (117.56), C7 (140.7 ppm), C1 (146.22 ppm), C2 (113.9 ppm), C3 (129.5 ppm), C4 (122.8 ppm), C5 (129.5 ppm) and C6 (113.9 ppm).
Compound 5: 4-((2-phenylhydrazono) methyl)pyridine: Yield 73%. m.p = 179–181 °C.¹HNMR (400 MHz, CDCl3): d 6.90-8.55 ppm (m, aromatic protons), 7.60 ppm (s, -CH-) and 8.15 (s, -NH- ).13CNMR (100 MHz, CDCl3): d C2 (149.98 ppm), C3 (120.13 ppm), C4 (143.47 ppm), C5 (120.13 ppm), C6 (149.98 ppm), C7 (142.84 ppm), C1 (133.55 ppm), C2 (113.09 ppm), C3 (129.42 ppm), C4 (121.13 ppm), C5 (129.42 ppm) and C6 (113.09 ppm).
Compound 6: (E)-1-(4-nitrobenzylidene)-2-phenylhydrazine: Yield 32.2%. m.p = 110–112 °C.1HNMR (400 MHz, CDCl3): d 6.80- 7.40 ppm (m, aromatic protons), 7.55 ppm (s, -CH-) and 9.88 ppm (s, -NH-).13CNMR (100 MHz, CDCl3): d C1 (147.18 ppm), C2 (119.06 ppm), C3 (119.84 ppm), C4 (144.93 ppm), C5 (119.84 ppm), C6 (119.06 ppm), C7 (137.29 ppm), C1 (145.85 ppm), C2 (111.66 ppm), C3 (129.28 ppm), C4 (112.71 ppm), C5 (129.28 ppm) and C6 (111.66 ppm).
Compound 7: (E)-1-(furan-2-ylmethylene)-2-phenylhydrazine: Yield 65%. m.p = 113–115 °C.IR:692.95, 743.06 cm-1 (aromatic, bending), 818.48 cm-1 (N-H, overtone), 1153.57 cm-1 (C-N), 1342.30 cm-1 (C-O), 1602.35 cm-1 (C=C, aromatic), 1604 cm-1 (N-H, bending), 1655 cm-1 (C=N), 2025 cm-1 (C-H, aromatic overtone), 3090 cm-1 (CH, aromatic) and 3317.56 cm-1 (N-H, stretching).1HNMR (400 MHz, CDCl3): d 6.85-7.55 ppm (m, aromatic protons), 7.60 ppm (s, -CH-) and 9.75 ppm (s, -NH-).13CNMR (100 MHz, CDCl3): d C2 (144.36 ppm), C3 (112.89 ppm), C4 (120.46 ppm), C5 (150.55 ppm), C6 (142.72 ppm), C1 (143 ppm), C2 (112.96 ppm), C3 (129.31 ppm), C4 (127.83 ppm), C5 (129.31 ppm) and C6 (112.96 ppm).
Compound 8: (E)-1-phenyl-2-((E)-3-phenylallylidene) hydrazine: Yield 80.5%. m.p = 150–152 °C.1HNMR (400 MHz, CDCl3): d 6.75 ppm (t,-CH-), 7.05 ppm (d,-CH-), 6.85-7.50 ppm (m, aromatic protons), 7.55 ppm (s,-CH-) and 9.75 ppm (s, -NH- ).13CNMR (100 MHz, CDCl3): d C1 (132.5 ppm), C2 (130 ppm), C3 (127 ppm), C4 (125 ppm), C5 (127 ppm), C6 (130 ppm), C7 (134 ppm), C8 (123 ppm), C9 (140 ppm), C1 (145 ppm), C2 (118 ppm), C3 (129 ppm), C4 (122 ppm), C5 (129 ppm) and C6 (118 ppm).
Compound 9: (E)-1-(4-chlorobenzylidene)-2-phenylhydrazine: Yield 80.1%. m.p = 119–121 °C.IR:691.09, 746.28 cm-1 (mono-sub.), 819.32 cm-1 (para-di-sub.) (aromatic, bending), 882.19 cm-1 (NH, overtone), 1133.08 cm-1 (C-N), 1518.02 cm-1 (N-H, bending), 1598.38 cm-1 (C=C, aromatic), 1620.02 cm-1 (C=N), 2000 cm-1 (C=C, aromatic), 3000 cm-1 (C-H, aromatic) and 3310.61 cm-1 (N-H, stretching).¹HNMR (400 MHz, CDCl3): d 6.95-7.50 ppm (m, aromatic protons), 7.90 ppm (s,-CH-) and 10.10 ppm (s, -NH-).13CNMR (100 MHz, CDCl3): d C1 (134.5 ppm), C2 (130.2 ppm), C3 (132.3 ppm), C4 (136.9 ppm), C5 (132.3 ppm), C6 (130.2 ppm), C7 (140.5 ppm), C1 (144.8 ppm), C2 (112 ppm), C3 (129.7 ppm), C4 (122.9 ppm), C5 (129 ppm) and C6 (112 ppm). MS: m/z: 230.06 (100.0%), (M+1) 231.10 (63.7%), (M+2) 229.05 (36.3%).
Compound 10: (E)-1-(4-bromobenzylidene)-2- phenylhydrazine: Yield 71%. m.p = 115–117 °C.1HNMR (400 MHz, CDCl3): d 7.0-7.60 ppm (m, aromatic protons), 7.98 ppm (s,-CH-) and 9.85 ppm (s, -NH-).13CNMR (100 MHz, CDCl3): d C1 (129.3 ppm), C2 (133.3 ppm), C3 (131.5 ppm), C4 (136.7 ppm), C5 (131.5 ppm), C6 (133.3 ppm), C7 (142.8 ppm), C1 (145.6 ppm), C2 (113.8 ppm), C3 (128 ppm), C4 (121.4 ppm), C5 (128 ppm) and C6 (113.8 ppm). MS: m/z: 276 (100.0%), (M+1) 278.95 (70 %), (M+2) 280.95 (30%).
Compound 11: 1,4-bis((2-phenylhydrazono)methyl)benzene: Yield 62%. m.p = 220–222 °C.IR:690.53, 743.71 cm-1 (aromatic, bending), 885.30 cm-1 (N-H, overtone), 1130.68 cm-1 (C-N), 1522.08 cm-1 (N-H, bending), 1588.48 cm-1 (C=C, aromatic), 1600.36 cm-1 (C=N), 1925.25 cm-1 (C-H, aromatic overtone), 3075.25 cm-1 (C-H, aromatic) and 3299.42 cm-1 (N-H, stretching).1HNMR (400 MHz, CDCl3): d 6.95-7.90 ppm (m, aromatic protons), 7.75 ppm (s, -CH-), 10.03 ppm (s,-NH-).13CNMR (100 MHz, CDCl3): d C1 (145 ppm), C2 (115 ppm), C3 (130 ppm), C4 (122 ppm), C5 (130 ppm), C6 (115 ppm), C7 (140 ppm), C8 (136 ppm), C9 (129 ppm), C10 (129 ppm), C11 (136 ppm), C12 (129 ppm), C11 (136 ppm), C12 (129 ppm), C13 (129 ppm), C14 (140 ppm), C15 (145 ppm), C16 (115 ppm), C17 (130 ppm), C18 (122 ppm), C19 (130 ppm) and C20 (115 ppm).
Results
Hot Plate Test Results
Control Standard drugs No Significance Significance High Significance.
Data are expressed as means ± SD of six mice per group.
Indomethacin (10 mg/kg), diclofenac sodium (7 mg/kg), tested compounds (100 mg/kg), was given orally one hour before carried out the experiment.
A significantly different from control group, using one-way ANOVA followed by Tukey-Kramer test for multiple comparisons at P ≤0.05.
Data are expressed as means ± SD of six mice per group.
Indomethacin (10 mg/kg), tested compounds (100 mg/kg), was given orally one hour before carried out the experiment.
a Significantly different from control group. Using one-way ANOVA followed by Tukey-Kramer test for multiple comparisons at P≤0.05.
Rat Paw Oedema Test Results
The injection of 0.1 ml saline in right hind paw (S.C) caused no significant increase in right paw volume by about (25.7%, 34.7%, 15.4% 6.5% 4.1% and 1.36% after 1, 2, 3, 6, 12 and 24 h respectively when compared to volume of left leg, while injection of 0.1 ml carrageenan in right hind paw (S.C) in a dose of 1 % w/v caused a significant increase in right paw volume by about (113.6%, 203.0%, 231.5% 204.0% 155.8% and 98.5% after 1, 2, 3, 6, 12 and 24 h respectively when compared to volume of left leg, while pretreatment with diclofenac sodium (I.P) in a dose of 7 mg/kg caused a significant reduction in right paw volume by (28.7%, 50.6%, 69.03%, 91.4%, 86.8% and 15.4%), when compared to volume of left leg at 1, 2, 3, 6, 12 and 24 h respectively. The pretreatment with indomethacin (orally) in a dose of 10 mg/kg caused a significant reduction in right paw volume by (25.3%, 46.6%, 63.5%, 67.2%, 55.5% and 17.5%), when compared to volume of left leg at 1, 2, 3, 6, 12 and 24 h respectively. The compounds 1, 4 and 6 at a dose of (200 mg/kg orally) shows no significant reduction in right hind paw volume when compared to carrageenan injected group at 1, 2, 3, 6, 12 and 24 h respectively taken into consideration that these compounds had no activity when compared to the two standards (diclofenac sodium and indomethacin). The use of tested compounds (2, 3, 7, 8, 9 and 10) at (200 mg/kg orally) had mild reduction in right paw volume when compared to volume of left leg at 1, 2, 3, 6, 12 and 24 h, respectively. The use of tested compounds (5 and 11) at (200 mg/kg orally) had a remarkable promising reduction in right paw volume when compared to volume of left leg at 1, 2, 3, 6, 12 and 24 h respectively.

Figure 4: Analgesic effects of Indomethacin, Diclofenac Sodium and tested
compounds (1-11) in comparison to control non-treated mice using hot plate
method.

Figure 5: The results of tested compounds (5,11) in anti-inflammatory activity test using rat paw oedema test.
Ulcer Index Test Results
Based on the results of hot plate test and rat paw oedema test, the results of ulcer index test are presented, as mentioned before compounds 3, 10 and 11 exhibited the best results as analgesic and anti-inflammatory agents. Concerning their gastric safety profiles, it was found to be that these compounds are way safer than the usage of indomethacin, which is clarified in (Figures 6-9).
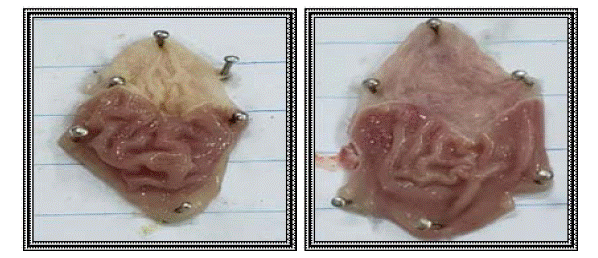
Figure 6: Normal stomach (control group) (group I).
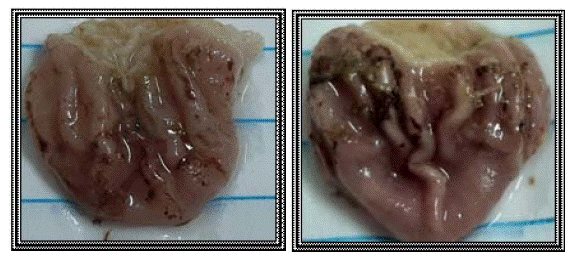
Figure 7: Indomethacin group (group II).

Figure 8: Compound 3 group (group V).
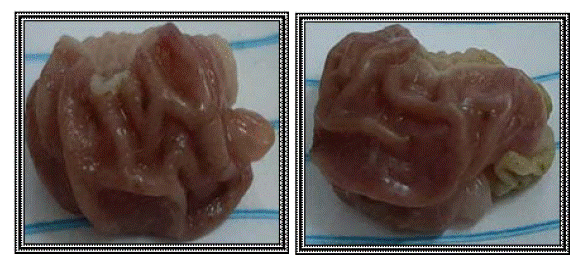
Figure 9: Compound 10 group (group XII).
Molecular Modelling of Some of the Tested Compounds
COX-2 enzyme is used as a target protein for docking, the molecular modelling study were performed using “Molecular Operating Environment” MOE software. The study for the tested compounds were performed in order to find the favorable binding configurations between ligands (compounds 3, 10 and 11) and the protein COX-2, COX-2 PDB code: 1PXX (25) which is illustrated in (Figures 11-13).

Figure 10: Compound 11 group (group XIII).

Figure 11: 2D, 3D docking model of compound 3 with COX-2 The depicted binding mode of compound 3 give rise to fact that the ligand contacted the receptor at
Ala 2527, Val 2349 and Leu 2531. The ligand exposed at the two phenyl groups. Leu 2531 performedhydrophobic interaction (p-alkyl) with phenyl group (Figure
11).
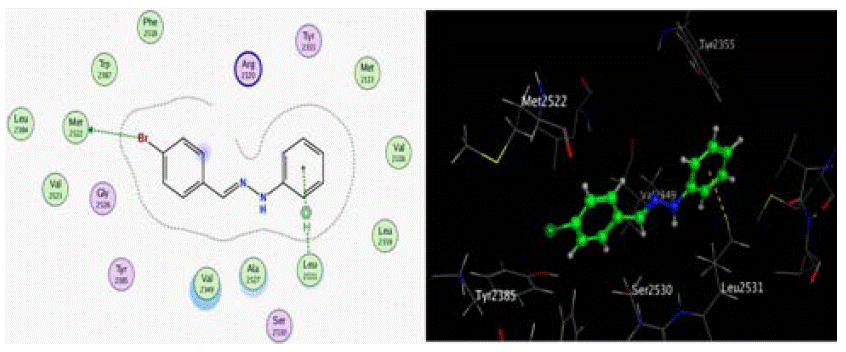
Figure 12: 2D, 3D docking model of compound 10 with COX-2 The illustrated binding mode of compound 10 showed that the ligand is exposed at the para
substituted phenyl moiety. Ligand contacted the receptor at Ala 2527, Leu 2531 and Val 2349. Leu 2531 performed hydrophobic interaction (p-alkyl interaction)
with the other phenyl group.
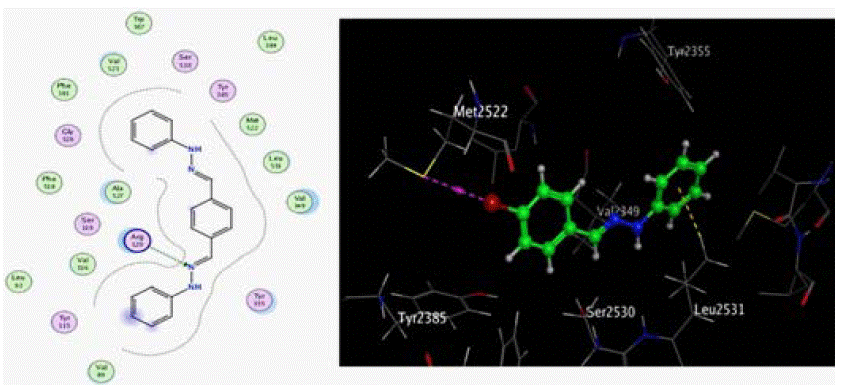
Figure 13: 2D, 3D docking model of compound 11 with COX-2 The shown binding mode of compound 11 showed that the ligand is exposed at two different phenyl
groups. Ligand contacted the receptor at Arg 120, Ala 527, Val 523, Tyr 355, Val 349 and Leu 531. Arg 120 performed hydrogen bonding with the benzylidene
moiety through the backbone of the amino acid.
Conclusion
For analgesic activity, the treatment with Indomethacin produced elevation in latency time also, Diclofenac Sodium produced a highly significant increase in latency time when compared to control animals. Compounds (3, 4, 5, 8, 9 and 10) showed a very highly significant increase in latency time, a moderate non-significant increase in response was observed during carried out the experiment with compound (1), finally there is a mild with no significant increase in latency time for the remaining compounds (2, 6, 7 and 11) when compared to non-treated animals (Table 1 & Figure 4).
Tested groups
Latency time (sec.)
Percentage increase in latency time
Control
10.17 ± 0.6
------------------
Indomethacin
18.00 ± 1.4a
77%
Diclofenac sod.
18.33 ± 2.7 a
80.20%
1
16.83 ± 0.9
61.10%
2
13.67 ±1.4
34.40%
3
19.50 ± 2.5 a
91.70%
4
18.50 ± 1.1 a
81.90%
5
17.50 ± 0.9 a
72.10%
6
14.67 ± 1.1
44.20%
7
14.50 ± 0.9
42.60%
8
17.67 ± 1.0 a
73.70%
9
17.17 ± 1.3 a
68.90%
10
19.50 ± 1.2 a
91.70%
10
13.17 ± 0.7
29.50%
Table 1: Analgesic effects of Indomethacin, Diclofenac Sodium and tested compounds (1-11) ((Figure 2,3) in comparison to control nontreated mice using hot plate method.
For anti-inflammatory activity, the selected dose (200 mg/kg) of tested compounds were evaluated in comparison to Diclofenac Sodium and Indomethacin as a reference drugs and were measured before and 1, 2, 3, 6, 12, and 24 h after carrageenan injection. Compounds (1, 4 and 6) had shown no anti-inflammatory activity at all-time points. Compounds (2, 3, 7, 8, 9 and 10) had shown mild antiinflammatory activity at all-time points, finally, Compounds (5 and 11) had shown highest anti-inflammatory activity at all-time points (Figure 5). Our study concluded that compounds (3, 5, 10 and 11) are the most potent compounds in as analgesic and anti-inflammatory agents with a very safe gastric safety profiles.
References
- Mahgoub MY, Elmaghraby AM, Harb AA, Galego F, Oliveira MC, Silva JLFD, et al. Synthesis and characterization of 2-arylidene derivatives of thiazolopyrimidines with potential biological activity. SCI Forum. 2016: 1.
- Ibrahim TH, Loksha YM, Elshihawy HA, Khodeer DM, Said MM. Synthesis of Some Novel 2,6-Disubstituted Pyridazin-3(2H)-one Derivatives as Analgesic, Anti-Inflammatory, and Non-Ulcerogenic Agents. Archiv der Pharmazie. 2017; 350: 1700093.
- Abouzid KAM, Khalil NA, Ahmed EM, Esmat A, Al-Abd AM. Design, synthesis, and evaluation of anti-inflammatory and ulcerogenicity of novel pyridazinone derivatives. Medicinal Chemistry Research. 2011; 21: 3581-3590.
- Saeed MM, Khalil NA, Ahmed EM, Eissa KI. Synthesis and anti-inflammatory activity of novel pyridazine and pyridazinone derivatives as non-ulcerogenic agents. Archives of Pharmacal Research. 2012; 35: 2077-2092.
- Zarraga IGE, Schwarz ER. Coxibs and heart disease: what we have learned and what else we need to know. Journal of the American College of Cardiology. 2007; 49: 1-14.
- Salem M, Ayyad R, Sakr H, Gaafer A. Design and Synthesis of New Compounds Derived from Phenyl Hydrazine and Different Aldehydes as Anticancer Agents. International Journal of Organic Chemistry. 2022; 12: 28- 39.
- Salem M, Ayyad R, Sakr H. Design and Synthesis of Some New Oxadiazole Derivatives as Anticancer Agents. International Journal of Organic Chemistry. 2022; 12: 64-74.
- Khalifa MM, Sakr HM, Ibrahim A, Mansour AM, Ayyad RR. Design and synthesis of new benzylidene-quinazolinone hybrids as potential anti-diabetic agents: In vitro a-glucosidase inhibition, and docking studies. Journal of Molecular Structure. 2021; 1250: 131768.
- El-Helby AA, Sakr H, Ayyad RR, Mahdy HA, Khalifa MM, Belal A, et al. Design, synthesis, molecular modeling, in vivo studies and anticancer activity evaluation of new phthalazine derivatives as potential DNA intercalators and topoisomerase II inhibitors. Bioorganic chemistry. 2020; 103: 104233.
- Eldehna WM, Abou-Seri SM, Kerdawy AME, Ayyad RR, Hamdy AM, Ghabbour HA, et al. Increasing the binding affinity of VEGFR-2 inhibitors by extending their hydrophobic interaction with the active site: Design, synthesis and biological evaluation of 1-substituted-4-(4-methoxybenzyl)phthalazine derivatives. European journal of medicinal chemistry. 2016; 113: 50-62.
- El-Helby A, Ayyad RR, Sakr HM, Abdul-Rahim AS. Design, synthesis, molecular modeling and biological evaluation of novel dihydrophthalazine-1, 4-dione derivatives as potential anticonvulsant agents. J Mol Struct. 2017; 1130: 333-351.
- El-Helby AA, Ayyad RRA, Sakr H, El-Adl K, Ali MM, Khedr F. Design, Synthesis, Molecular Docking, and Anticancer Activity of Phthalazine Derivatives as VEGFR-2 Inhibitors. Archiv der Pharmazie. 2017; 350: 1700240.
- El-Helby A, Ayyad RR, El-Adl K, El-Kady H. Phthalazine-1, 4-dione derivatives as non-competitive AMPA receptor antagonists: design, synthesis, anticonvulsant evaluation, ADMET profile and molecular docking. Molecular Diversity. 2019; 23: 283-298.
- Eissa IH, Metwaly AM, Belal A, Mehany ABM, Ayyad RR, El-Adl K, et al. Discovery and antiproliferative evaluation of new quinoxalines as potential DNA intercalators and topoisomerase II inhibitors. Archiv der Pharmazie. 2019; 352: 1900123.
- El-Helby A, Ayyad RR, Zayed MF. Synthesis and biological evaluation of some novel quinoxaline derivatives as anticonvulsant agents. Arzneimittelforschung. 2011; 61: 379-381.
- El-Helby AA, Ayyad RRA, El-Adl K, Elwan A. Quinoxalin-2(1H)-one derived AMPA-receptor antagonists: Design, synthesis, molecular docking and anticonvulsant activity. Medicinal Chemistry Research. 2017; 26: 2967-2984.
- El-Helby AA, Ayyad RRA, Zayed MF, Abulkhair HS, Elkady H, El-Adl K. Design, synthesis, in silico ADMET profile and GABA-A docking of novel phthalazines as potent anticonvulsants. Archiv der Pharmazie. 2019; 352: 1800387.
- El-Helby AA, Sakr H, Ayyad RRA, El-Adl K, Ali MM, Khedr F. Design, Synthesis, In Vitro Anti-cancer Activity, ADMET Profile and Molecular Docking of Novel Triazolo[3,4-a]phthalazine Derivatives Targeting VEGFR-2 Enzyme. Anti-cancer agents in medicinal chemistry. 2018; 18: 1184-1196.
- Ekhlass MN, Abdul Razek FM, Ayyad RR, El-Farargy AF. Synthesis and some reactions of 1-aryl-4-acetyl-5-methyl-1, 2, triazole derivatives with anticonvulsant activity. Mini-Rev Med Chem. 2016; 16: 926-36.
- Ayyad RR, Sakar HM, El-Gammal K. Synthesis, modeling and anticonvulsant activity of some phthalazinedione. Am J Org Chem. 2016; 6, 29-38.
- Eddy NB, Leimbach DJ. Synthetic analgesics. II. Dithienylbutenyl and dithienylbutylamines. Journal of Pharmacology and Experimental Therapeutics. 1953; 107: 385-393.
- Ostertagova E, Ostertag O. Methodology and Application of One-way ANOVA. American Journal of Mechanical Engineering. 2013; 1: 256-261.
- Cong HH, Khaziakhmetova VN, Zigashina LE. Rat paw edema modeling and NSAIDs: Timing of effects. Int J Risk Saf Med. 2015; 27: 76-86.
- Sattar A, Abdo A, Mushtaq MN, Anjum I, Anjum A. Evaluation of Gastroprotective Activity of Myristica fragrans on Ethanol-induced Ulcer in Albino Rats. Anais da Academia Brasileira de Ciencias. 2019; 91: e20181044.
- Rowlinson SW, Kiefer JR, Prusakiewicz JJ, Pawlitz JL, Kozak KR, Kalgutkar AS, et al. A Novel Mechanism of Cyclooxygenase-2 Inhibition Involving Interactions with Ser-530 and Tyr-385. Journal of Biological Chemistry. 2003; 278: 45763-45769.