Review Article
Austin J Anat. 2014;1(1): 1003.
Aetiological Factors for Developmental Defects of Enamel
Hai Ming Wong*
Department of Dentistry, University of Hong Kong, Hong Kong
*Corresponding author: Hai Ming Wong, Department of Dentistry, University of Hong Kong, The Prince Philip Dental Hospital, 34 Hospital Road, Hong Kong SAR.
Received: May 19, 2014; Accepted: May 21, 2014; Published: May 22, 2014
Abstract
Developmental defects of enamel remain as a permanent record of a disturbance during amelogenesis. They may present in different forms, some of which may be perceived by an individual as being disfiguring and so requiring treatment to improve the appearance of the teeth. The aim of this review is to address the aetiological factors for DDE because the knowledge is essential for clinicians when explaining and discussing the presence of DDE with patients and their parents. The possible aetiological factors for enamel defects in permanent teeth can be broadly divided into two main categories: those with a localized distribution and those with a generalized distribution. Amongst the causative agents of localized defects of enamel are trauma, localized infection and irradiation. Amongst the causative agents of generalized defects of enamel are genetic disorders and systemic disturbances including intoxications, perinatal and postnatal problems, malnutrition, infectious diseases and a range of other medical conditions. Most of the available data on the aetiology of enamel defects have been gained from animal studies and case reports of children with systemic disorders. The lack of robust data makes the results of these studies inconclusive.
Keywords: Developmental defects of enamel; Aetiological factors
Abbreviations
DDE: Developmental Defects of Enamel
Introduction
Tooth enamel is formed during only a certain period of the tooth development and is irreplaceable. Ameloblasts, which are secretory cells that produce dental enamel, are particularly sensitive to changes in their environment during the long process of enamel production. Dysfunction of ameloblasts may occur resulting in changes in the appearance of the enamel in the permanent dentition. These Developmental Defects of Enamel (DDE) may range from slight abnormalities of the tooth’s colour to a complete absence of the enamel.
Impacts of DDE
Effects of DDE may include tooth sensitivity or an increased risk of caries. Treatment of DDE attempts to improve the function and appearance of the affected teeth [1]. There is evidence that teeth with DDE have 10 times greater treatment need than normal teeth [2]. Apart from financial considerations of dental treatment, there is also the social cost including children’s absence from school and parents’ absence from work to attend multiple appointments. An affected individual may also experience low self–esteem or stigma because they perceive DDE as being disfiguring [3,4]. The knowledge of aetiological factors for DDE is essential for clinicians when explaining and discussing the presence of DDE with patients and their parents. Targeting risk factors could also assist in implementation of community strategies to limit the occurrence of DDE.
Terminology of DDE
An early report of enamel defects, according to Sarnat and Schour [5], appeared over 200 years ago when rickets, measles and scurvy were said to be associated with ‘erosion’ of the teeth. The term ‘mottled enamel’ was adopted by Black and McKay [6] to describe the appearance of teeth which they considered to represent an endemic form of the defect; it was not until 1931 that fluoride was identified as the causative agent of this defect [7]. The examples of the terminology that have been used in published studies to describe developmental defects of enamel are shown in Table 1 [5,6,8–20]. Some are simple descriptive clinical terms, while others are linked with the causative agent, or the histopathology of the defect. However, the majority of these terms are non–specific and frequently ambiguous. The terminology needs to be uniform to suit the requirements of the various investigators. Owing to the efforts of a working group of the Commission on Oral Health, Research and Epidemiology of the International Dental Federation (FDI), a standardized terminology,which accompanies the FDI (DDE) Index, has been established [21,22]. Based on the quality and quantity of affected enamel, DDE can be classified into three main types: demarcated opacities diffuse opacities and hypoplasia [22].
Terminology
Author
Chalky enamel
Gottlieb [8]
Mottling
Black and McKay [6], Dean [9]
Chronologic enamel aplasia
Sarnat and Schour [5], [10]
Hypoplasia, hypocalcification
Weinmann [11]
Opaque hypoplasia
Hurme [12]
Fluorosed and idiopathic opacities
Zimmermann [13]
Hypoplasia, mottling, pigmentation
Hewat and Eastcott [14]
Fluorosed and non-endemic mottling
Jackson [15]
Internal and external hypoplasia
Andreasen and Ravn [16]
Opacities, hypoplasia
Young [17]
Opacities, pits, grooves
Suckling [18]
Cheese molar
Weerheijm [19]
Molar incisor hypomineralization (MIH)
Weerheijm [20]
Table 1: Examples of the terminology that have been used in some published studies to describe DDE.
Pathology of DDE
The stage of amelogenesis at which time the dysfunction occurs, the severity of the insult leading to temporary, or permanent inactivity of the cells, the duration of the insult, the phase of ameloblast activity during the relevant period, and the specific agent involved, may affect the final appearance of the defect [23,24]. For example, damage of secretory ameloblasts results in pathologically thin enamel. However, interference during the maturation stage can lead to defects which present as bands, or patches of chalky opaque porous enamel [25]. This is due to failure of the degradation and removal of some amino acid fractions; therefore, relatively large residues of organic material remain between the crystals. The presence of an organic ‘contaminant’ can retard or arrest further growth of the apatite crystals that are already present [26,27]. Moreover, Hall [28] claimed that some ameloblasts might subsequently recover from the insult during the maturation phase, which may account for the varying colour and degree of mineralization of the opacities that are known to occur. Different agents might initiate common pathways which lead to enamel defects with a similar appearance. Furthermore, based upon work on sheep, Suckling [23] suggested that the pathogenesis of each type of DDE was different and therefore should be considered separately.
Pathology of demarcated opacities
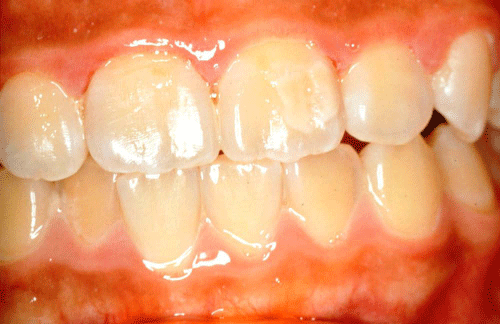
The demarcated opacity is a defect which involves an alteration in the translucency and has a distinct and clear boundary with the adjacent normal enamel, Figure 1. The colour can be white, cream, yellow or brown. Demarcated opacities have been produced experimentally in sheep. This defect followed trauma to cells in either the early or late maturation phase [29,30], and after parasitic infection in the secretory or early maturation phases [31]. Suckling claimed that yellow demarcated opacities resulted from an insult causing death of the ameloblasts early in their maturation stage while white demarcated opacities were found in sheep incisors following disturbances in the secretory, plus the early– and the late–maturation phases. Suckling also claimed that the yellow demarcated opacities that she induced often had a white opaque margin, which had a higher hardness value than normal enamel. This leads to the assumption that some maturation cells as well as secretory cells have the ability to recover [32].
Figure 1: Cream demarcated opacities on the upper left central incisor.
However, work on the teeth of humans and monkeys by Suga [33], suggested that ameloblasts were very sensitive to disorders at an early stage of maturation. Hence, if a cell is damaged by a systemic or local disorder at this stage, it cannot easily recover from dysfunction during the long period of maturation. Therefore, he hypothesized that demarcated opacities were due to a disturbance in the process of matrix degradation, which originally occurs during the matrix formation stage, to provide suitable physiochemical conditions for the commencement of maturation.
In respect of the severity and duration of the insult, Suckling [23] presumed that demarcated opacities were caused by a less severe, but longer lasting disturbance than that responsible for causing hypoplasia. Birgitta and Jörgen [24] observed that the homologous teeth of children with demarcated opacities were affected to varying extents. However, they found it difficult to make any assumption about the severity of the insult because the damaging agent seemedto have been rather nonspecific in most of the children. Hence, they assumed that two or more interacting factors were required toproduce the defects [24].
Pathology of diffuse opacities
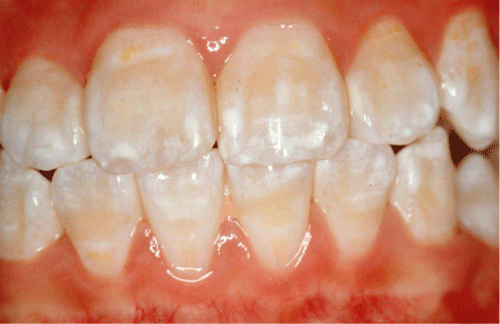
Diffuse opacities also involve alterations in the translucency of enamel and are white in colour, Figure 2. They can have a linear, patchy or confluent distribution but there is no clear boundary with the adjacent normal enamel. Histologically diffuse opacities are subsurface–hypo mineralized defects covered by a well–mineralized outer enamel surface. It is believed that these defects result from along, continuous low–grade insult. They have been produced in sheep by a daily low dose of fluoride for a period of six months [34]. Diffuse opacities have been associated with an arrest in enamel maturation characterized by delayed breakdown of amelogenins [35], which may become entrapped in the detective enamel. Moreover, data from a sheep study showed that the defects increased in severity when fluoride was also given during the secretory phase [34]. However, if the insult was confined to the secretory phase, then normal maturation occurred, resulting in translucent enamel even if the matrix was abnormal [36].
Figure 2: Diffuse opacities.
The effect of increasing the intake of fluoride during tooth development upon the appearance of the enamel has been welldocumented. Among the many hypotheses that have been proposed for the mechanism by which excess fluoride affects degradation and removal of enamel matrix proteins, three are favored by most researchers: (i) fluoride might directly affect ameloblasts [37]; (ii) proteins may be more tightly bound to fluoridated hydroxyapatite and, thus, proteinolysis might be more difficult [38]; and (iii) fluoride might inhibit enamel proteinases [39]. However, there is no conclusive proof that fluoride alone, either in the form of an excessive intake, or of an abnormal metabolic process in the presence of a low or normal intake, is responsible for all the enamel changes of diffuse opacities. Other systemic factors operating over a long period of tooth development such as malnutrition [40], chronic illness (diabetes insipidus) [41], or high altitude [42] may mimic or augment the effects of fluoride. If the defects are true fluorosis, then it is an apt reminder that biological factors can enhance cellular responses to low concentrations of fluoride in the environment [23].
Pathology of hypoplasia
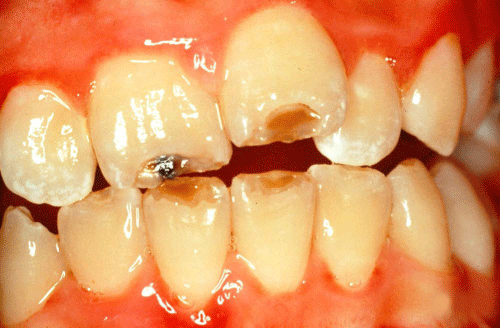
Hypoplasia is a defect associated with a reduced quantity and hence thickness of enamel, Figure 3. Sarnat and Schour [5,10] believed that the morphologic unit of enamel hypoplasia was the enamel pit, which resulted from a cessation of ameloblastic activity. In a clinical investigation of anxious patients, whose childhood medical histories were available, it was concluded that a narrow zone of defect indicated a disturbance of short duration or an acute disease, while a wide zone indicated a disturbance of long duration or chronic disease [5,10].
Figure 3: Hypoplasia on the incisal edges of the four incisors.
More recently enamel hypoplasia has been produced in sheep by physical trauma [29,30], by systemic illness induced by intestinal parasites [31,43], and by a daily high dose of fluoride for a short period [44,45]. These findings indicate that hypoplastic defects areformed during the secretory phase of amelogenesis. The duration of the insult is relatively short, and it is the severity that determines the extent of the defect and the translucency of the partially formed enamel. The aetiology does not seem to be of major importance, since local and systemic factors result in defects with a similar appearance and physical characteristics.
The chronological course of tooth development and the occurrence of DDE
The formation of the crowns of the 28 permanent teeth commences at around birth and is completed at around age 8 years [46]. Only dysfunction of ameloblasts during this period of time may result in the occurrence of DDE. Furthermore, enamel, once formed, has limited capacity for alteration. Therefore, enamel can serve as a kymograph which permits the dating of events that occurred during amelogenesis with a high degree of accuracy [5,10].
Location of DDE
The location of DDE depends on the stage of enamel production and on the time of the insult or injury to the ameloblasts. As enamel production commences at varying times in different tooth types, so the location of DDE will vary in different homologous pairs of teeth. All the teeth at the same stage of development may be affected, with homologous pairs of teeth having similar types of DDE in similar locations. This type of distribution is referred to as generalized DDE and may be caused by systemic factors. When only one or several adjacent teeth exhibit the same type of DDE, the defect causing event is probably localized.
Aetiology of DDE
Developmental defects of enamel with a similar appearance are not necessarily caused by similar aetiological agents. Conversely, the same aetiological factors can produce different defects at different stages of tooth development. Enamel defects may also result from a combination of factors. It has been proposed that there are well over 90 different factors that may be responsible for causing developmental defects of enamel [47,48]. Most of the available data on the aetiology of enamel defects have been gained from animal studies and case reports of children with systemic disorders; however, sound evidence for their involvement are equivocal. Only a few of these factors have been confirmed as being directly responsible for causing developmental defects. The possible aetiological factors for DDE in permanent teeth can be broadly divided into two main categories: those with a localized distribution and those with a generalized distribution.
Possible aetiological factors for DDE with a localized distribution
When one, or several, adjacent teeth in a horizontal, or vertical relationship exhibit enamel defect, the defect causing event was probably of a localized, rather than a generalized nature [49]. The localized disturbance, experienced by the individual, affected only a small group of ameloblasts. Amongst the causative agents of localized defects of enamel are trauma, localized infection and irradiation.
Trauma
Trauma in the form of a fall, or a blow to a primary tooth, causing intrusion or lateral luxation, are probably the most common causes of localized enamel defects in the succedaneous teeth [50–53]. The damage might happen either at the time of injury, by direct impact of the root apex of the primary incisor on the developing permanent tooth, or at a later stage, as a consequence of post–traumatic complications. If the trauma to primary incisors leads to pulp necrosis then there is a greater likelihood of enamel defects occurring in the permanent successors [54].
Regrettably, another cause of trauma is surgery. In an Australian study, Williamson [55] demonstrated that exodontias could be a factor in the production of enamel defects in developing premolars. Children with cleft palates have significantly higher prevalence of enamel defects in primary as well as in permanent teeth [56]. These defects predominantly occur adjacent to the site of the surgical repair of the lip and palate. Therefore, the defects may have resulted from trauma during surgery [56,57]. However, the effect of other postnatal environmental factors such as nutrition and infection should not be excluded [57].
Localized infection
In a cohort of Chinese children, higher prevalence’s of demarcated opacities and hypoplasia were found in permanent teeth whose primary predecessor teeth had experienced caries than in those without caries [58]. Caries extending into the pulp of primary teeth often results in necrosis of the pulp tissue and periapical lesions. In an animal study, Valderhaug [59] found that the chronic periapical periodontitis of primary tooth could destroy the cortical plate of bone surrounding the permanent tooth germ and affect the dental organ in various ways. Inter–radicular infection of a primary tooth can, in extreme cases, cause arrest of the developing tooth germ [60]. In a study conducted by McCormick and Filostrat [61], over 25% of abscessed primary teeth were associated with enamel defects, which varied from opacities to hypoplasia, in the successional permanent teeth. This phenomenon has also been observed by other researchers [62–66].
Irradiation
Since anti–neoplastic therapy affects all cells, it is not surprising that developmental defects of enamel have been documented after oncology therapy [67–72]. In the study conducted by Pajari and Lanning [73], it was found that the scattered irradiation, of 0.72– 1.44Gy, to the dental arches during central nervous system irradiation caused an increase in the number of enamel opacities in children with acute lymphoblastic leukemia. In another study, Duggal and his colleagues [74] reported a significantly higher prevalence of all types of enamel defects and fewer teeth without enamel defects in the long term survivors of childhood cancer as compared with their siblings.
Possible aetiological factors for DDE with a generalized distribution
Developmental defects with generalized type of distribution may be caused by genetic disorders, or by environmental factors.
Genetic disorders
Amelogenesis Imperfecta (AI) has been defined as a heterogeneous group of genetic disorders affecting the enamel of the teeth by causing various degrees of hypoplasia, hypo mineralization or, a combination of the two [75,76]. The condition often results from a single gene defect, either as an X–linked, autosomal dominant or autosomal recessive trait [77–79]. Several classifications have evolved since 1945, based on the phenotype and mode of inheritance [75,80–83]. Nevertheless, some authors consider that AI may be present as part of hereditarily determined syndrome complexes, such as epidermolysis bullosa [84,85], pseudohypoparathyroidism [86], and tricho–dentoosseous syndrome [87]. Genetically determined enamel defects are less common than those resulting from acquired causes; for example, AI has been estimated to vary in the general population from about 1:14,000 [88] to less than 1:800 [89].
Some genetically–determined syndromes and disorders, as well as some acquired diseases, are frequently accompanied by enamel defects. The Finnish researcher Aine [90,91] found that more than 90% of patients with coeliac disease had enamel defects. She also claimed that the so–called coeliac–type enamel defect, with associated gastrointestinal symptoms, could lead to the diagnosis of coeliac disease [92]. Confirming Aine’s results, Farmakis [93] and colleagues and Avsar and colleagues [94] also found higher percentage of enamel defects among celiac disease patients compared with the healthy controls. Other researchers have, however, failed to find any association between enamel defects and coeliac disease [95–97]. In tuberous sclerosis, pit–shaped enamel defects were said to be pathogenomonic of the syndrome [98]; however, this has been questioned [99,100] because other researchers have found only a few cases of enamel pitting among tuberous sclerosis families [101]. By studying children with hereditary vitamin D resistant rickets, X–linked hypophosphatemia and hypoparathyroidism, it has been hypothesized that the enamel hypoplasia was caused by hypocalcemia. This observation led to formulation of a unifying hypothesis that enamel hypoplasias are caused specifically by hypocalcemia [102,103]. Other inherited disorders which have been reported, in the literature, to possibly be accompanied by enamel defects are shown in Table 2 [85–87,90,91,98,102–122].
Hereditary condition
Author
Auto-immune polyendocrinopathy-candidiasis
-ectodermal dystrophy
Lukinmaa [104]
Candidasisendocrinopathy syndrome
Porter [105]
Cleidocranaldysostosis
Fukuta [106]
Coeliac disease
Aine [90,91]
Congenital adrenal hyperplasia
Hallett and Hall [107]
Congenital contractual arachnodactyly
Ayers and Drummond [108]
Congenital unilateral facial hypoplasia
Gibbard and Winter [109]
Ectodermal dysplasias
Sastry [110]
Ehlers-Danlos syndrome
Letournean [111]
Epidermolysisbullosa
Wright [85]
Focal dermal hypoplasia
Al-Ghamdi and Crawford [112]
Heimler’s syndrome
Pollak [113]
Hypoparathyroidism
Nikiforuk and Fraser [102,103]
Ichthyosis vulgaris
Pindborg [114]
Lacrimo-ariculo-dento-digital syndrome
Hollister [115]
Morguio syndrome
Barker and Welbury [116]
Mucopolysaccharidosis
Gorlin [117]
Oculodentodigital dysplasia
Frasson [118]
Orodigitofacialdysostosis
Gorlin [117]
Prader-Willi syndrome
Jablonski [119]
Pseudohypoparathyroidism
Jensen [86]
Seckel syndrome
Seymen [120]
Tricho-dento-osseous syndrome
Spangler [87]
Tuberous sclerosis
Hoff [98]
Vitamin D resistant rickets
Nikiforuk and Fraser [102,103]
William’s syndrome
Hertzberg [121]
22q11 deletion syndrome
Klingberg [122]
Table 2: Hereditary conditions that have been reported to be associated with DDE.
Environmental factors
A considerable number of environmental factors have been reported to be capable of causing enamel defects. These systemic disturbances consist of intoxications, perinatal and postnatal problems, malnutrition, infectious diseases and a range of other medical conditions.
Intoxications
In the group of intoxications, the one responsible for affecting most people is probably fluoride. The relationship between DDE and fluoride consumption has been established for over 80 years and is well documented [7,9]. The prevalence of enamel defects increases with increasing levels of fluoride in the drinking water [123]. Fluorotic defects can vary from minor white striations to small or extensive areas of opaque enamel [124,125]. However, fluorotic defects do not have unique characteristics which allow them to be differentiated from defects caused by other factors [126,127]. The level of strontium in the drinking water has been shown to be associated with the frequency and severity of enamel mottling similar to that of fluorosis [128]. It seems that, the less severe the defects, the greater the problem of positive diagnosis for fluorosis [129,130].
Recently, Finnish studies have focused on dioxins as being a causative agent for developmental defects of permanent first molars [131,132]. Prolonged breast–feeding might increase mineralization defects in teeth because of environmental contaminants such as dioxins or dioxin–like compounds in breast milk [131,132] interfering with enamel maturation [133]. However, a Swedish study which identified similar levels of dioxins in breast milk failed to reproduce the findings [134]. Wozniak [135] reported that elevated levels of chemical compounds, e.g. fluorine, ammonia and sulphur, in the atmosphere increased the incidence of diffuse white⁄creamy mottling and lines in the 14 to 15 years old schoolchildren. Other intoxications include hypervitaminosis D [114], chronic lead poisoning [136], diphosphonate [137], and polychlorinated biphenyl poisoning [138].
Perinatal and postnatal problems
Improvements in the medical care of neonates and infants have improved the survival rates of low birth–weight and premature babies. However, they are prone to suffer from many serious illnesses, which in turn may cause the development of enamel defects through a central metabolic mechanism [139,140]. Children with low birthweights, i.e. 2000g or below, have also been shown to have a much higher prevalence of enamel opacities in the first permanent molars and lateral incisors than children who had a normal birth–weight [141].
Medical problems at the time of delivery such as breech presentation, caesarean section and labour in excess of 20 hours, as well as a poor respiratory response in the postnatal period have been said to be associated with enamel hypoplasia of the primary dentition [142]. In a Dutch study of potential aetiological factors responsible for mineralization defects of permanent first molars, the authors noted a high frequency of medical problems at the time of delivery and childhood respiratory diseases [143]. According to their findings, it was suggested that oxygen deprivation influenced the mineralization of enamel matrix. However, Beentjes and his co–workers [144] found that there was no relation between enamel defects and complications during pregnancy and birth.
Malnutrition
Medical problems at the time of delivery such as breech presentation, caesarean section and labour in excess of 20 hours, as well as a poor respiratory response in the postnatal period have been said to be associated with enamel hypoplasia of the primary dentition [142]. In a Dutch study of potential aetiological factors responsible for mineralization defects of permanent first molars, the authors noted a high frequency of medical problems at the time of delivery and childhood respiratory diseases [143]. According to their findings, it was suggested that oxygen deprivation influenced the mineralization of enamel matrix. However, Beentjes and his co–workers [144] found that there was no relation between enamel defects and complications during pregnancy and birth.
Infectious diseases and other medical conditions
Infectious diseases during early childhood, such as chicken–pox, measles, mumps, scarlet fever [150], tuberculosis [100], pneumonia, diphtheria, whooping cough [151], otitis media [152], and bulbar polio with encephalitis [153], have long been blamed for the presence of enamel defects. A number of other medical conditions such asgastro–intestinal disturbances [154], cyanotic congenital heart disease [155], neurological disorders [156], and renal disorder [157] have also been implicated as being aetiological agents. However, except for chicken–pox, Suckling and her co–workers [52] failed to find positive and strong associations between enamel defects and children experiencing one or more of these diseases in spite of extensive statistical testing.
Jackson [15] considered that the exanthematous fevers were a major cause of non–endemic mottling of the permanent first molars but only a contributory factor in other teeth. However, Wilson and Cleaton–Jones [158] found no association between the presence of enamel mottling and the occurrence of childhood fever in subjects from a fluoride–deficient area. In the study conducted by Suckling and Pearc [149], trauma and ‘serious illness’ were the only two aetiological factors which were statistically significant in a cohort of 243 children. ‘Serious illness’ in their study included urinary tract infections, convulsions and pneumonia. In one Australian study [159], infection was reported to be related to demarcated enamel defects. Swedish researchers suggested that respiratory diseases, especially asthma, were possible aetiological factors responsible for demarcated opacities in first molars; however, only four of the 516 subjects suffered from asthma [134]. The limited studies that involved human populations were predominately retrospective; moreover, their conclusions can be criticized because only a small number of subjects had developmental defects with an associated aetiology. Hence, the lack of robust data makes the results of these studies inconclusive.
One notable exception involved a group of New Zealand children who participated in the Dunedin Multidisciplinary Child Developmental Study [160]. This longitudinal study correlated the medical and dental histories of 696 children born in 1972 with the presences of enamel defects using the FDI (DDE) Index [21]. It was found that, apart from exposure to fluoride, chicken–pox before the age of 3 years and trauma to the primary teeth were the only other significant factors. However, the severity of systematic infections in these children is likely to have been reduced by immunization, the use of antibiotics and readily available medical facilities [52]. Nevertheless, this study demonstrates the difficulty of establishing the aetiology of enamel defects, even when medical and dental histories are available and when a study is prospective. Thus, the paucity of reliable information on the possible causal factors in the occurrence of DDE suggests a need for further investigations into the causes ofthe defects.
Conclusion
Because of the nature of DDE, the difficulty of methodology establishing the aetiology of enamel defects, lack of robust data, and the limitation of this paper, i.e. non–systematic review, the results of the reviewed studies are inconclusive. To obtain reliable data for aetiological factors of DDE, a cohort observational epidemiologic study in an area with high prevalence of DDE is needed. A longterm prospective study of a large group of young subjects using the same strict diagnostic criteria for DDE, and with frequent updates to health–related events in their early childhood, is the only realistic way to gather the necessary data on the aetiological factors of DDE.
Acknowledgement
The conduction this literature review was fully supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China [Project No. 782811].
References
- Wong HM, McGrath C, King NM. Dental practitioners' views on the need to treat developmental defects of enamel. Community Dent Oral Epidemiol. 2007; 35: 130-139.
- Jälevik B, Klingberg GA. Dental treatment, dental fear and behaviour management problems in children with severe enamel hypomineralization of their permanent first molars. Int J Paediatr Dent. 2002; 12: 24-32.
- McKnight CB, Levy SM, Cooper SE, Jakobsen JR. A pilot study of esthetic perceptions of dental fluorosis vs. selected other dental conditions. ASDC J Dent Child. 1998; 65: 233-238, 229.
- Wondwossen F, Astrøm AN, BÅrdsen A, Bjorvatn K. Perception of dental fluorosis amongst Ethiopian children and their mothers. Acta Odontol Scand. 2003; 61: 81-86.
- Sarnat BG, Schour I. Enamel hypoplasia (chronologic enamel aplasia) in relation to systemic disease: a chronologic, morphologic and etiologic classification (part I). J Am Dent Assoc. 1941; 28: 1989-2000.
- Black GV, McKay F. Mottled teeth. An endemic developmental imperfection of the dental enamel of the teeth, heretofore unknown in the literature of dentistry. Dental Cosmos. 1916; 58: 129-156.
- Churchill HV. Occurrence of fluorides in some waters of the United States. IndEng Chem. 1931; 23: 996-998.
- Gottlieb B. Rachitis and enamel hypoplasia. Dental Cosmos. 1920; 62: 1316-1326.
- Dean HT. Classification of mottled enamel diagnosis. J Am Dent Assoc. 1934; 21: 1421-1426.
- Sarnat BG, Schour I. Enamel hypoplasia (chronologic enamel aplasia) in relation to systemic disease: a chronologic, morphologic and etiologic classification (part II). J Am Dent Assoc. 1942; 29: 67-75.
- Weinmann JP, Svoboda JF, Woods RW. Hereditary disturbances of enamel formation and calcification. J Am Dent Assoc. 1945; 32: 397-418.
- HURME VO. Developmental opacities of teeth in a New England community; their relation to fluorine toxicosis. Am J Dis Child. 1949; 77: 61-75.
- ZIMMERMANN ER. Fluoride and nonfluoride enamel opacities. Public Health Rep. 1954; 69: 1115-1120.
- Hewat RET, Eastcott DF. Dental caries in New Zealand. Medical Research Council Report. 1955.
- JACKSON D. A clinicla study of non-endemic mottling of enamel. Arch Oral Biol. 1961; 5: 212-223.
- Andreasen JO, Ravin JJ. Enamel changes in permanent teeth after trauma to their primary predecessors. Scand J Dent Res. 1973; 81: 203-209.
- Young MA. An epidemiological study of enamel opacities. PhD Thesis, University of London 1973.
- Suckling GW, Pearce EI, Cutress TW. Developmental defects of enamel in New Zealand children. N Z Dent J. 1976; 72: 201-210.
- Weerheijm KL, Groen HJ, Beentjes VE, Poorterman JH. Prevalence of cheese molars in eleven-year-old Dutch children. ASDC J Dent Child. 2001; 68: 259-262, 229.
- Weerheijm KL, Jälevik B, Alaluusua S. Molar-incisor hypomineralisation. Caries Res. 2001; 35: 390-391.
- [No authors listed]. An epidemiological index of developmental defects of dental enamel (DDE Index). Commission on Oral Health, Research and Epidemiology. Int Dent J. 1982; 32: 159-167.
- [No authors listed]. A review of the developmental defects of enamel index (DDE Index). Commission on Oral Health, Research & Epidemiology. Report of an FDI Working Group. Int Dent J. 1992; 42: 411-426.
- Suckling GW. Developmental defects of enamel--historical and present-day perspectives of their pathogenesis. Adv Dent Res. 1989; 3: 87-94.
- Jälevik B, Norén JG. Enamel hypomineralization of permanent first molars: a morphological study and survey of possible aetiological factors. Int J Paediatr Dent. 2000; 10: 278-289.
- Moss-Salentijn L, Hendricks-Klyvert M. Dental and oral tissue: an introduction. 2nd edition. Lea &Febiger:Philadelphia. 1990.
- Fearnhead RW, Kawasaki K, Inoue K. Comments on the porosity of human tooth enamel. J Dent Res. 1982; Spec No: 1524-1531.
- Robinson C, Kirkham J, Briggs HD, Atkinson PJ. Enamel proteins: from secretion to maturation. J Dent Res. 1982; Spec No: 1490-1495.
- Hal RK. The role of CT, MRI and 3D imaging in the diagnosis of temporomandibular joint and other orofacial disorders in children. Aust Orthod J. 1994; 13: 86-94.
- Suckling G. Defects of enamel in sheep resulting from trauma during tooth development. J Dent Res. 1980; 59: 1541-1548.
- Suckling GW, Purdell-Lewis DJ. The pattern of mineralization of traumatically-induced developmental defects of sheep enamel assessed by microhardness and microradiography. J Dent Res. 1982; 61: 1211-1216.
- Suckling G, Elliott DC, Thurley DC. The production of developmental defects of enamel in the incisor teeth of penned sheep resulting from induced parasitism. Arch Oral Biol. 1983; 28: 393-399.
- Suckling GW, Nelson DG, Patel MJ. Macroscopic and scanning electron microscopic appearance and hardness values of developmental defects in human permanent tooth enamel. Adv Dent Res. 1989; 3: 219-233.
- Suga S. Enamel hypomineralization viewed from the pattern of progressive mineralization of human and monkey developing enamel. Adv Dent Res. 1989; 3: 188-198.
- Suckling GW, Thurley DC, Nelson NGA. The macroscopic and scanning electron microscopic appearance and microhardness of the enamel, and the related histological changes in the enamel organ of erupting sheep incisors resulting from a prolonged low daily dose of fluoride. Arch Oral Biol. 1988; 33: 361-373.
- Den Besten PK. Effects of fluoride on protein secretion and removal during enamel development in the rat. J Dent Res. 1986; 65: 1272-1277.
- Purdell-Lewis DJ, Suckling GW, Triller M, Jongebloed WL . Artificially induced developmental defects in sheep enamel examined by scanning electron microscopy. J Biol Buccale. 1987; 15: 119-124.
- Denbesten PK, Crenshaw MA, Wilson MH. Changes in the fluoride-induced modulation of maturation stage ameloblasts of rats. J Dent Res. 1985; 64: 1365-1370.
- Tanabe T, Aoba T, Moreno EC, Fukae M. Effect of fluoride in the apatitic lattice on adsorption of enamel proteins onto calcium apatites. J Dent Res. 1988; 67: 536-542.
- DenBesten PK, Heffernan LM. Enamel proteases in secretory and maturation enamel of rats ingesting 0 and 100 PPM fluoride in drinking water. Adv Dent Res. 1989; 3: 199-202.
- Rugg-Gunn AJ, al-Mohammadi SM, Butler TJ. Effects of fluoride level in drinking water, nutritional status, and socio-economic status on the prevalence of developmental defects of dental enamel in permanent teeth in Saudi 14-year-old boys. Caries Res. 1997; 31: 259-267.
- Seow WK, Thomsett MJ. Dental fluorosis as a complication of hereditary diabetes insipidus: studies of six affected patients. Pediatr Dent. 1994; 16: 128-132.
- Rwenyonyi C, Bjorvatn K, Birkeland J, Haugejorden O. Altitude as a risk indicator of dental fluorosis in children residing in areas with 0.5 and 2.5 mg fluoride per litre in drinking water. Caries Res. 1999; 33: 267-274.
- Suckling G, Elliott DC, Thurley DC. The macroscopic appearance and associated histological changes in the enamel organ of hypoplastic lesions of sheep incisor teeth resulting from induced parasitism. Arch Oral Biol. 1986; 31: 427-439.
- Suckling GW, Purdell-Lewis DJ. Macroscopic appearance, microhardness and microradiographic characteristics of experimentally produced fluorotic lesions in sheep enamel. Caries Res. 1982; 16: 227-234.
- Suckling G, Thurley DC. Histological, macroscopic and microhardness observations of fluoride-induced changes in the enamel organ and enamel of sheep incisor teeth. Arch Oral Biol. 1984; 29: 165-177.
- Fejerskov O, Josephen K. In: Human oral embryology and histology. Mjör IA, Fejerskov O, editors. Munksgaard: Copenhagen. 1986: 31-49.
- Small BW, Murray JJ. Enamel opacities: prevalence, classifications and aetiological considerations. J Dent. 1978; 6: 33-42.
- Pindborg JJ. Aetiology of developmental enamel defects not related to fluorosis. Int Dent J. 1982; 32: 123-134.
- Jorgenson RJ, Yost C. Etiology of enamel dysplasia. J Pedod. 1982; 6: 315-329.
- Hall SR, Iranpour B. The effect of trauma on normal tooth development. Report of two cases. ASDC J Dent Child. 1968; 35: 291-295.
- Andreasen JO, Sundström B, Ravn JJ. The effect of traumatic injuries to primary teeth on their permanent successors. I. A clinical and histologic study of 117 injured permanent teeth. Scand J Dent Res. 1971; 79: 219-283.
- Suckling GW, Herbison GP, Brown RH. Etiological factors influencing the prevalence of developmental defects of dental enamel in nine-year-old New Zealand children participating in a health and development study. J Dent Res. 1987; 66: 1466-1469.
- Sleiter R, von Arx T. [Developmental disorders of permanent teeth after injuries of their primary predecessors. A retrospective study]. Schweiz Monatsschr Zahnmed. 2002; 112: 214-219.
- Holan G, Topf J, Fuks AB. Effect of root canal infection and treatment of traumatized primary incisors on their permanent successors. Endod Dent Traumatol. 1992; 8: 12-15.
- Williamson JJ. Trauma during exodontia. An aetiologic factor in hypoplastic premolars. Br Dent J. 1966; 121: 284-289.
- Dixon DA. Defects of structure and formation of the teeth in persons with cleft palate and the effect of reparative surgery on the dental tissues. Oral Surg Oral Med Oral Pathol. 1968; 25: 435-446.
- Ranta R. A review of tooth formation in children with cleft lip/palate. Am J Orthod Dentofacial Orthop. 1986; 90: 11-18.
- Lo EC, Zheng CG, King NM. Relationship between the presence of demarcated opacities and hypoplasia in permanent teeth and caries in their primary predecessors. Caries Res. 2003; 37: 456-461.
- Valderhaug J. Periapical inflammation in primary teeth and its effect on the permanent successors. Int J Oral Surg. 1974; 3: 171-182.
- Brook AH, Winter GB. Developmental arrest of permanent tooth germs following pulpal infection of deciduous teeth. Br Dent J. 1975; 139: 9-11.
- McCormick J, Filostrat DJ. Injury to the teeth of succession by abscess of the temporary teeth. J Dent Child. 1967; 34: 501-504.
- Turner JG. Two cases of hypoplasia of enamel. Proc R Soc Med 1912; 5: 73-76.
- BAUER WH. Effect of periapical processes of deciduous teeth on the buds of permanent teeth; pathological-clinical study. Am J Orthod Oral Surg. 1946; 32: 232-241.
- Binns WH Jr, Escobar A. Defects in permanent teeth following pulp exposure of primary teeth. J Dent Child. 1967; 34: 4-14.
- Kaplan NL, Zach L, Goldsmith ED. Effects of pulpal exposure in the primary dentition on the succedaneous teeth. J Dent Child. 1967; 34: 237-242.
- Kimoto S, Suga H, Yamaguchi M, Uchimura N, Ikeda M, Kakizawa T. Hypoplasia of primary and permanent teeth following osteitis and the implications of delayed diagnosis of a neonatal maxillary primary molar. Int J Paediatr Dent. 2003; 13: 35-40.
- Welbury RR, Craft AW, Murray JJ, Kernahan J. Dental health of survivors of malignant disease. Arch Dis Child. 1984; 59: 1186-1187.
- Pajari U, Lanning M, Larmas M. Prevalence and location of enamel opacities in children after anti-neoplastic therapy. Community Dent Oral Epidemiol. 1988; 16: 222-226.
- Purdell-Lewis DJ, Stalman MS, Leeuw JA, Humphrey GB, Kalsbeek H. Long term results of chemotherapy on the developing dentition: caries risk and developmental aspects. Community Dent Oral Epidemiol. 1988; 16: 68-71.
- Sonis AL, Tarbell N, Valachovic RW, Gelber R, Schwenn M, Sallan S. Dentofacial development in long-term survivors of acute lymphoblastic leukemia. A comparison of three treatment modalities. Cancer. 1990; 66: 2645-2652.
- Nunn JH, Welbury RR, Gordon PH, Kernahan J, Craft AW. Dental caries and dental anomalies in children treated by chemotherapy for malignant disease: a study in the north of England. Int J Paediatr Dent. 1991; 1: 131-135.
- Minicucci EM, Lopes LF, Crocci AJ. Dental abnormalities in children after chemotherapy treatment for acute lymphoid leukemia. Leuk Res. 2003; 27: 45-50.
- Pajari U, Lanning M. Developmental defects of teeth in survivors of childhood ALL are related to the therapy and age at diagnosis. Med Pediatr Oncol. 1995; 24: 310-314.
- Duggal MS, Curzon ME, Bailey CC, Lewis IJ, Prendergast M. Dental parameters in the long-term survivors of childhood cancer compared with siblings. Oral Oncol. 1997; 33: 348-353.
- Winter GB, Brook AH. Enamel hypoplasia and anomalies of the enamel. Dent Clin North Am. 1975; 19: 3-24.
- Collins MA, Mauriello SM, Tyndall DA, Wright JT. Dental anomalies associated with amelogenesis imperfecta: a radiographic assessment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88: 358-364.
- Wright JT, Hart PS, Aldred MJ, Seow K, Crawford PJ, Hong SP, Gibson CW. Relationship of phenotype and genotype in X-linked amelogenesis imperfecta. Connect Tissue Res. 2003; 44 Suppl 1: 72-78.
- Gopinath VK, Al-Salihi KA, Yean CY, Ann MC, Ravichandran M. Amelogenesis imperfecta: enamel ultra structure and molecular studies. J Clin Pediatr Dent. 2004; 28: 319-322.
- Kim JW, Simmer JP, Hu YY, Lin BP, Boyd C, Wright JT, Yamada CJ. Amelogenin p.M1T and p.W4S mutations underlying hypoplastic X-linked amelogenesis imperfecta. J Dent Res. 2004; 83: 378-383.
- Weinmann JP, Svoboda JF, Woods RW. Hereditary disturbances of enamel formation and calcification. J Am Dent Assoc. 1945; 32: 397-418.
- Sundell S, Koch G. Hereditary amelogenesis imperfecta. I. Epidemiology and clinical classification in a Swedish child population. Swed Dent J. 1985; 9: 157-169.
- Witkop CJ Jr. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. J Oral Pathol. 1988; 17: 547-553.
- Aldred MJ, Savarirayan R, Crawford PJ. Amelogenesis imperfecta: a classification and catalogue for the 21st century. Oral Dis. 2003; 9: 19-23.
- ARWILL T, OLSSON O, BERGENHOLTZ A. EPIDERMOLYSIS BULLOSA HEREDITARIA. 3. A HISTOLOGIC STUDY OF CHANGES IN TEETH IN THE POLYDYSPLASTIC DYSTROPHIC AND LETHAL FORMS. Oral Surg Oral Med Oral Pathol. 1965; 19: 723-744.
- Wright JT, Johnson LB, Fine JD. Development defects of enamel in humans with hereditary epidermolysis bullosa. Arch Oral Biol. 1993; 38: 945-955.
- Jensen SB, Illum F, Dupont E. Nature and frequency of dental changes in idiopathic hypoparathyroidism and pseudohypoparathyroidism. Scand J Dent Res. 1981; 89: 26-37.
- Spangler GS, Hall KI, Kula K, Hart TC, Wright JT. Enamel structure and composition in the tricho-dento-osseous syndrome. Connect Tissue Res. 1998; 39: 165-175.
- WITKOP CJ. Hereditary defects in enamel and dentin. Acta Genet Stat Med. 1957; 7: 236-239.
- Bäckman B, Holmgren G. Amelogenesis imperfecta: a genetic study. Hum Hered. 1988; 38: 189-206.
- Aine L. Dental enamel defects and dental maturity in children and adolescents with coeliac disease. Proc Finn Dent Soc. 1986; 82: 1-71.
- Aine L. Coeliac-type permanent-tooth enamel defects. Ann Med. 1996; 28: 9-12.
- Aine L. Permanent tooth dental enamel defects leading to the diagnosis of coeliac disease. Br Dent J. 1994; 177: 253-254.
- Farmakis E, Puntis JW, Toumba KJ. Enamel defects in children with coeliac disease. Eur J Paediatr Dent. 2005; 6: 129-132.
- AvÅäar A, Kalayci AG. The presence and distribution of dental enamel defects and caries in children with celiac disease. Turk J Pediatr. 2008; 50: 45-50.
- Andersson-Wenckert I, Blomquist HK, Fredrikzon B. Oral health in coeliac disease and cow's milk protein intolerance. Swed Dent J. 1984; 8: 9-14.
- Raether D, Klingberg G, Magnusson L, Norén JG. Histology of primary incisor enamel in children with early onset celiac disease. Pediatr Dent. 1988; 10: 301-303.
- Rasmusson CG, Eriksson MA. Celiac disease and mineralisation disturbances of permanent teeth. Int J Paediatr Dent. 2001; 11: 179-183.
- Hoff M. A dental aspect of tuberous sclerosis [proceedings]. Br J Dermatol. 1980; 102: 476.
- Bhat M, Nelson KB. Developmental enamel defects in primary teeth in children with cerebral palsy, mental retardation, or hearing defects: a review. Adv Dent Res. 1989; 3: 132-142.
- Flanagan N, O'Connor WJ, McCartan B, Miller S, McMenamin J, Watson R. Developmental enamel defects in tuberous sclerosis: a clinical genetic marker? J Med Genet. 1997; 34: 637-639.
- Fleury P, De Groot WP, Delleman JW, Verbeeten B, Frankenmolen-Witkiezwicz IM. Tuberous sclerosis. The incidence of sporadic cases versus family cases. Brain Dev. 1980; 2: 107-117.
- Nikiforuk G, Fraser D. Aetiology of enamel hypoplasia and interglobular dentin: The roles of hypocalcemia and hypophosphatemia. Metab Bone Dis Relat. 1979; 2: 17-23.
- Nikiforuk G, Fraser D. The etiology of enamel hypoplasia: a unifying concept. J Pediatr. 1981; 98: 888-893.
- Lukinmaa PL, Waltimo J, Pirinen S. Microanatomy of the dental enamel in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED): report of three cases. J Craniofac Genet Dev Biol. 1996; 16: 174-181.
- Porter SR, Eveson JW, Scully C. Enamel hypoplasia secondary to candidiasis endocrinopathy syndrome: case report. Pediatr Dent. 1995; 17: 216-219.
- Fukuta Y, Totsuka M, Fukuta Y, Takeda Y, Yoshida Y, Niitsu J, et al. Histological and analytical studies of a tooth in a patient with cleidocranialdysostosis. J Oral Sci. 2001; 43: 85-89.
- Hallett KB, Hall RK. Congenital adrenal hyperplasia and enamel hypoplasia: case report. Pediatr Dent. 1995; 17: 54-59.
- Ayers KM, Drummond BK. Novel dental anomalies associated with congenital contractural arachnodactyly: a case report. Pediatr Dent. 2003; 25: 501-504.
- Gibbard PD, Winter GB. Congenital unilateral facial hypoplasia associated with dental abnormalities. A case report. Br Dent J. 1972; 132: 404-406.
- Sastry KA, Ruprecht A, Suliman AM. Hypoplastic enamel, onycholysis and hypohydrosis: a report of two cases. J Oral Med. 1983; 38: 21-23.
- Létourneau Y, Pérusse R, Buithieu H. Oral manifestations of Ehlers-Danlos syndrome. J Can Dent Assoc. 2001; 67: 330-334.
- Al-Ghamdi K, Crawford PJ. Focal dermal hypoplasia -- oral and dental findings. Int J Paediatr Dent. 2003; 13: 121-126.
- Pollak C, Floy M, Say B. Sensorineural hearing loss and enamel hypoplasia with subtle nail findings: another family with Heimler's syndrome. Clin Dysmorphol. 2003; 12: 55-58.
- Pindborg JJ. Pathology of the dental hard tissues. Saunders: Philadelphia. 1970.
- Hollister DW, Klein SH, De Jager HJ, Lachman RS, Rimoin DL. The lacrimo-auriculo-dento-digital syndrome. J Pediatr. 1973; 83: 438-444.
- Barker D, Welbury RR. Dental findings in Morquio syndrome (mucopolysaccharidoses type IVa). ASDC J Dent Child. 2000; 67: 431-433, 407.
- Gorlin RJ, Pindborg JJ, Cohen MM. Syndromes of the head and neck. 2nd ed. McGraw-Hill Book Company: New York. 1976.
- Frasson M, Calixto N, Cronemberger S, de Aguiar RA, Leao LL, de Aguiar MJ. Oculodentodigital dysplasia: study of ophthalmological and clinical manifestations in three boys with probably autosomal recessive inheritance. Ophthalmic Genet. 2004; 25: 227-236.
- Jablonski S. Illustrated Dictionary of Dentistry. Saunders: Philadelphia. 1982; 779-780.
- Seymen F, Tuna B, Kayserili H. Seckel syndrome: report of a case. J Clin Pediatr Dent. 2002; 26: 305-309.
- Hertzberg J, Nakisbendi L, Needleman HL, Pober B. Williams syndrome--oral presentation of 45 cases. Pediatr Dent. 1994; 16: 262-267.
- Klingberg G, Oskarsdóttir S, Johannesson EL, Norén JG. Oral manifestations in 22q11 deletion syndrome. Int J Paediatr Dent. 2002; 12: 14-23.
- Wong HM, McGrath C, Lo EC, King NM. Association between developmental defects of enamel and different concentrations of fluoride in the public water supply. Caries Res. 2006; 40: 481-486.
- Fejerskov O, Larsen MJ, Richards A, Baelum V. Dental tissue effects of fluoride. Adv Dent Res. 1994; 8: 15-31.
- Rozier RG. Epidemiologic indices for measuring the clinical manifestations of dental fluorosis: overview and critique. Adv Dent Res. 1994; 8: 39-55.
- Cutress TW, Suckling GW, Pearce EI, Ball ME. Defects of tooth enamel in children in fluoridated and non-fluoridated water areas of the Auckland region. N Z Dent J. 1985; 81: 12-19.
- Cutress TW, Suckling GW. Differential diagnosis of dental fluorosis. J Dent Res. 1990; 69 Spec No: 714-720.
- Curzon ME, Spector PC. Enamel mottling in a high strontium area of the U.S.A. Community Dent Oral Epidemiol. 1977; 5: 243-247.
- Møller IJ. Fluorides and dental fluorosis. Int Dent J. 1982; 32: 135-147.
- Driscoll WS, Horowitz HS, Meyers RJ, Heifetz SB, Kingman A, Zimmerman ER. Prevalence of dental caries and dental fluorosis in areas with optimal and above-optimal water fluoride concentrations. J Am Dent Assoc. 1983; 107: 42-47.
- Alaluusua S, Lukinmaa PL, Koskimies M, Pirinen S, Hölttä P, Kallio M, et al. Developmental dental defects associated with long breast feeding. Eur J Oral Sci. 1996; 104: 493-497.
- Alaluusua S, Lukinmaa PL, Vartiainen T, Partanen M, Torppa J, Tuomisto J. Polychlorinated dibenzo-p-dioxins and dibenzofurans via mother's milk may cause developmental defects in the child's teeth. Environ Toxicol Pharmacol. 1996; 1: 193-197.
- Gao Y, Sahlberg C, Kiukkonen A, Alaluusua S, Pohjanvirta R, Tuomisto J, Lukinmaa PL. Lactational exposure of Han/Wistar rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin interferes with enamel maturation and retards dentin mineralization. J Dent Res. 2004; 83: 139-144.
- Jälevik B, Norén JG, Klingberg G, Barregård L. Eetiologic factors influencing the prevalence of demarcated opacities in permanent first molars in a group of Swedish children. Eur J Oral Sci. 2001; 109: 230-234.
- WoÅ°niak K. [Developmental abnormalities of mineralization in populations with varying exposure to fluorine compounds]. Ann Acad Med Stetin. 2000; 46: 305-315.
- Lawson BF, Stout FW, Ahern DE, Sneed WD. The incidence of enamel hypoplasia associated with chronic pediatric lead poisoning. S C Dent J. 1971; 29: 5-10.
- Fouda N, Caracatsanis M, Hammarström L. Developmental disturbances of the rat molar induced by two diphosphonates. Adv Dent Res. 1989; 3: 234-240.
- Jan J, Vrbic V. Polychlorinated biphenyls cause developmental enamel defects in children. Caries Res. 2000; 34: 469-473.
- Lai PY, Seow WK, Tudehope DI, Rogers Y. Enamel hypoplasia and dental caries in very-low birthweight children: a case-controlled, longitudinal study. Pediatr Dent. 1997; 19: 42-49.
- Seow WK. Effects of preterm birth on oral growth and development. Aust Dent J. 1997; 42: 85-91.
- Seow WK. A study of the development of the permanent dentition in very low birthweight children. Pediatr Dent. 1996; 18: 379-384.
- VIA WF Jr, CHURCHILL JA. Relationship of enamel hypoplasia to abnormal events of gestation and birth. J Am Dent Assoc. 1959; 59: 702-707.
- van Amerongen WE, Kreulen CM. Cheese molars: a pilot study of the etiology of hypocalcifications in first permanent molars. ASDC J Dent Child. 1995; 62: 266-269.
- Beentjes VE, Weerheijm KL, Groen HJ. Factors involved in the aetiology of molar-incisor hypomineralisation (MIH). Eur J Paediatr Dent. 2002; 3: 9-13.
- GRAHNEN H, SELANDER P. The effect of rickets and spasmophilia on the permanent dentition. I. The effect on the teeth. Odontol Revy. 1954; 5: 7-26.
- Goodman JR, Gelbier MJ, Bennett JH, Winter GB. Dental problems associated with hypophosphataemic vitamin D resistant rickets. Int J Paediatr Dent. 1998; 8: 19-28.
- Punyasingh JT, Hoffman S, Harris SS, Navia JM. Effects of vitamin A deficiency on rat incisor formation. J Oral Pathol. 1984; 13: 40-51.
- Berdal A, Balmain N, Cuisinier-Gleizes P, Mathieu H. Histology and microradiography of early post-natal molar tooth development in vitamin-D deficient rats. Arch Oral Biol. 1987; 32: 493-498.
- Suckling GW, Pearce EI. Developmental defects of enamel in a group of New Zealand children: their prevalence and some associated etiological factors. Community Dent Oral Epidemiol. 1984; 12: 177-184.
- Marshall JA. Dental hypoplasia: its occurrence, histopathology and aetiology. J Am Dent Assoc. 1936; 23: 2074-2082.
- GIRO CM. Enamel hypoplasia in human teeth; an examination of its causes. J Am Dent Assoc. 1947; 34: 310-317.
- STEIN G. Enamel damage of systemic origin in premature birth and diseases of early infancy. Am J Orthod. 1947; 33: 831-841.
- Alexander WN. Enamel hypoplasia. Oral Surg Oral Med Oral Pathol. 1966; 22: 338-339.
- Lindemann G. Prevalence of enamel hypoplasia among children that previously suffered from gastrointestinal disease. OdontTidskr. 1958; 66: 101-126.
- Hakala PE. Dental and oral changes in congenital heart disease. Suom Hammaslaak Toim. 1967; 63: 284-324.
- Cohen HJ, Diner H. The significance of developmental dental enamel defects in neurological diagnosis. Pediatrics. 1970; 46: 737-747.
- Woodhead JC, Nowak AJ, Crall JJ, Robillard JE. Dental abnormalities in children with chronic renal failure. Pediatr Dent. 1982; 4: 281-285.
- Wilson RM, Cleaton-Jones P. Enamel mottling and infectious exanthemata in a rural community. J Dent. 1978; 6: 161-165.
- Arrow P. Risk factors in the occurrence of enamel defects of the first permanent molars among schoolchildren in Western Australia. Community Dent Oral Epidemiol. 2009; 37: 405-415.
- Silva PA. The Dunedin Multidisciplinary Health and Development Study: a 15 year longitudinal study. Paediatr Perinat Epidemiol. 1990; 4: 76-107.