Editorial
Austin J Anat. 2014;1(2): 1007.
The Sources of Synthetic Vascular Smooth Muscle Cells Revisited
Leach DF, Mathis BJ, Qu C, Lai Y and Cui T*
Department of Cell Biology and Anatomy, University of South Carolina School of Medicine, USA
*Corresponding author: Cui T, Department of Cell Biology and Anatomy, University of South Carolina School of Medicine, Columbia, SC 29208, USA.
Received: May 22, 2014; Accepted: May 26, 2014; Published: May 26, 2014
Editorial
Vascular disease is the leading cause of mortality in the United States, and is characterized by abnormal growth of Vascular Smooth Muscle Cells (VSMCs). Extensive studies of vascular biology have shown mature VSMCs to be quiescent and contractile in normal vasculature; however, in response to injury, another unique type of VSMCs (i.e., synthetic VSMCs) becomes apparent [1]. These cells expand in number rapidly, releasing various cytokines and growth factors, all of which results in a vascular lesion. Although they may play a critical role in lesion formation, the origin of these synthetic VSMCs has been unanswered.
For more than 50 years, a widely accepted view is that, in response to vascular injury, these proliferative and synthetic VSMCs are derived from the dedifferentiation or phenotypic modulation of mature contractile VSMCs in the tunica media [2]. Other documented sources of synthetic VSMCs include bone marrow-derived stem cells and resident vascular stem cells [3–5]. However, it is clear that the differentiation of bone marrow–derived stem cells into VSMCs is an exceedingly rare event and the contribution of bone marrow–derived cells to the cellular compartment of the vascular lesion is limited to the transient inflammatory response phase [6]. Furthermore, synthetic VSMCs in a vascular lesion are exclusively derived from within the local vessel wall [6–8]. Therefore, synthetic VSMCs are reported to be likely derived from the dedifferentiation of mature VSMCs and⁄ or resident vascular stem cells. However, the relative contributions of mature VSMC dedifferentiation and resident Vascular Stem Cell (VSC) differentiation to the generation of synthetic VSMCs in the vasculature remain unknown.
Intriguingly, emerging evidence indicates that, upon endovascular injury, mature VSMCs at the wound site die and a small population (<10%) of media stem cells are activated, proliferate, and differentiate to intimal VSMCs, thereby contributing the vascular disease [9]. These cells, named media–derived Multipotent Vascular Stem Cells (MVSCs), were found in the media of mature blood vessels. They are Smooth Muscle–Myosin Heavy Chain (SM–MHC) negative (−), Stem Cell Antigen (Sca)–1−, neural crest cell marker Sry–box (Sox)10 positive (+), endoderm marker Sox17+, neural cell marker Neural Filament–Medium polypeptide (NFM)+, glia cell marker S100β)+, and general mesenchymal stem cell marker CD29+ and CD44+. These MVSCs have multilineage potential and can spontaneously differentiate into SMClike cells that subsequently differentiate intoVSMCs. Additionally, MVSCs and MSC–like cells have a differential response to the treatment of vascular growth factors bFGF, platelet–derived growth factors–β (PDGF–β) and transforming growth factor–β1 (TGF–β1).Importantly, utilizing SM–MHC–Cre⁄LoxP–EGFP mice generated using both ROSA26 and β–actin promoters to drive EGFP expression, the authors showed that the majority of enzymatically isolated carotid media cells are EGFP+; however, all these cells become EGFP– after being cultured and passaged for 10 days. These results indicate that proliferative⁄synthetic SMCs are not derived from mature VSMCs.In addition, it is the MVSCs, rather than mature VSMCs, that are activated, proliferate, repopulate the tunica media, and constitute the cellular population of a vascular lesion after injury. While this novel “MVSC” differentiation theory has been under intense scrutiny and skepticism [2], we have observed that the primary culture of rat thoracic aortic SMCs (RASMCs) is most likely a process of MVSC differentiation into SMCs (unpublished data). Of note, a very recent report also demonstrated that RASMCs express MVSC biomarkers and have the potential to differentiate into adipocytes and osteoblasts [10]. Collectively, even though this “MVSC” theory still needs conditional SMC lineage tracing to determine the ultimate fate of VSMCs, it has challenged the long–standing dogma of SMC de–differentiation in the process of vascular remodeling. These novel findings raise the question of whether vascular disease is in fact an SMC disease or a stem cell disease.
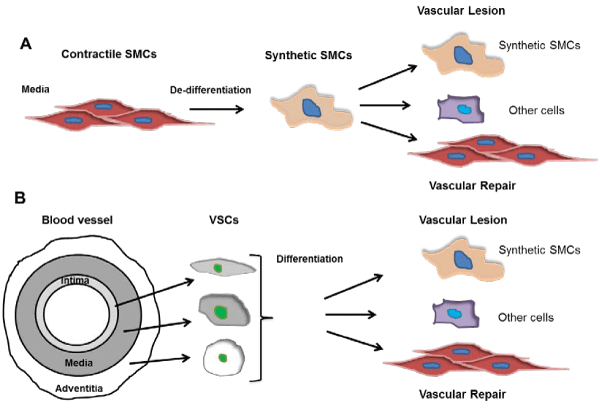
Figure 1: An emerging diagram of SMC de-differentiation and differentiation in the vasculature. A. The long-standing SMC de-differentiation theory. In response to injuries, media mature contractile SMCs de-differentiate into synthetic SMCs, and further differentiate into contractile SMCs or other cells such as adipocyte and osteocytes in diverse pathophysiological settings, contributing to vascular remodeling. B. Vascular stem cell (VSC) differentiation theory. VSCs represent important sources of synthetic SMCs and are found in all three layers of the blood vessel. After vascular injury, media mature SMCs die while VSCs differentiate into SMCs or other cell types, contributing to vascular repair or lesion formation.
Much work remains to be done. The identity and location of MVSCs need further investigation, and the definitive identification of modulated SMC phenotypes during vascular remodeling is still enigmatic. As the debates between the MVSC and SMC dedifferentiation theories (Figure 1) are going [2,11], the ambiguities regarding SMC de–dedifferentiation, as well as SMC– or other cell type–differentiation of MVSCs or other VSCs in vascular remodeling will be clarified.
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014; 129: e28-e292.
- Nguyen AT, Gomez D, Bell RD, Campbell JH, Clowes AW, Gabbiani G, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013; 112: 17-22.
- Orlandi A, Bennett M. Progenitor cell-derived smooth muscle cells in vascular disease. Biochem Pharmacol. 2010; 79: 1706-1713.
- Bautch VL. Stem cells and the vasculature. Nat Med. 2011; 17: 1437-1443.
- Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011; 50: 304-311.
- Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010; 30: 1890-1896.
- Atkinson C, Horsley J, Rhind-Tutt S, Charman S, Phillpotts CJ, Wallwork J, et al. Neointimal smooth muscle cells in human cardiac allograft coronary artery vasculopathy are of donor origin. J Heart Lung Transplant. 2004; 23: 427-435.
- Jevon M, Ansari TI, Finch J, Zakkar M, Evans PC, Shurey S, et al. Smooth muscle cells in porcine vein graft intimal hyperplasia are derived from the local vessel wall. Cardiovasc Pathol. 2011; 20: e91-94.
- Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012; 3: 875.
- Kennedy E, Hakimjavadi R, Greene C, Mooney CJ, Fitzpatrick E, Collins LE, et al. Embryonic rat vascular smooth muscle cells revisited - a model for neonatal, neointimal SMC or differentiated vascular stem cells? Vasc Cell. 2014; 6: 6.
- Tang Z, Wang A, Wang D, Li S. Smooth muscle cells: to be or not to be? Response to Nguyen et Al. Circ Res. 2013; 112: 23-26.