
Research Article
Austin J Anat. 2015;2(1): 1032.
Lower Lumbar Facet Joint Complex Anatomy
Gorniak G and Conrad W*
Department of Physical Therapy, University of St Augustine for Health Sciences, USA
*Corresponding author: Conrad W, Department of Physical Therapy, University of St Augustine for Health Sciences, 1 University Blvd, St Augustine, Fl. 32086-5783,USA.
Received: December 16, 2014; Accepted:March 16, 2015 Published: March 17, 2015
Abstract
Low back pain is often associated with osteoarthritis of the lower lumbar facet joints. Each facet joint has several structural components that interact to form a spinal segmental movement complex. The components of this complex are usually studied independently. In this descriptive cadaver study, the anatomy of the joint capsule and its relationship with the multifidus are described and compared to other studies. The sizes of the boney facets and the ligamentum flava of L3 – S1are compared for gender, sidedness and to other studies. Finally, the interaction of these components of the joint complex, relative to joint motion and wear, are discussed.
Keywords: Lumbar facet joints; Joint capsule; Ligamentum flava
Introduction
Osteoarthritis of the lumbar facet joints is a common source of low back pain and its prevalence increases with age [1-9]. It has been reported that as many as 89% -95% of individuals 65 and older have varying degrees of Facet Joint Arthritis (FJAO) and the L4-5 and L5-S1 are the most commonly seen [4,5,8,10]. From 2000 -2011, facet joint interventions in the Medicare population have increased by 308% per 100,000 beneficiaries and lumbosacral interventions by 544% per 100,000 [10]. As the population in the United States continues to age, further studies relating to lumbar facet joint degeneration and ageing changes are needed.
The anatomy of the lumbar facet joint has been studied macroscopically, microscopically and radiologically. L3/L4, L4/ L5, and L5/S1 have received the most attention. An overview of these studies indicates that the facet joint is an interacting complex of structures, working as a unit to produce functional segmental movements. The components of the lumbar facet complex are the articulating facets, the facet joint capsule, the ligamentum flavum and the multifidus muscle. Most studies tend to focus on one individual component of this facet complex, although, osteoarthritis of lumbar facet joints and other facet joint disorders may result from a disruption in the interactions of all these components of the joint complex. In this study, the roles of the joint capsule, ligamentum flavum and multifidus in regards to facet motion and wear are discussed.
The size, shape, and orientation of the lumbar facets have been described, as well as several have examined differences in these descriptors with age, gender and side [4,11-17]. The anatomy of the lumbar facet joint capsule has also been studied by many [1,18-26]. These studies describe different layers of the joint capsule, different collagen fiber directions, varying capsular thickness, menisci, subcapsular pockets and type I and type II capsular attachment patterns. Because most of these studies are microscopic and concentrate mainly on the fibrous capsule, the macroscopic relationships between the overlying ligamantum flavum and multifidus muscle needs clarification. Anatomical studies on the elastic ligamentum flavum describe its fiber composition, attachment sites, size, mechanical features and function [1,2,14,27-33]. The anatomy of the lumbar multifidus has also been described, and debated, but its actions on the spine are somewhat controversial [1,34-40].
The collagen arrangement of lumbar joint capsules and the configuration of the matching facets are associated with joint motion and joint dysfunctions [1,19,20,41-44]. The collagen fiber arrangement of a capsule may permit motion in one direction, but limit motion in another. Dysfunctional joint movements can result in excessive joint articular cartilage wear. Tightness of the capsule will limit motion and increase forces on the joint surfaces. This condition may result in joint pain and excessive articular surface wear. Stretched or torn capsules will increase motion, and may also produce articular damage because of abnormal motion and joint stress. Several studies describe lumbar facet movements, stresses on the joint surfaces and capsule, and the role of the joint capsule in limiting movement [1,6,18-21,43,45,46]. While these studies provide a good basic understanding of lumbar joint mechanics, the role of the facet complex on joint movement, and articular surface wear and damage need further investigation.
In this study, the sizes of the lumbar facets from L3 – S1 are described and compared with age, gender, side and spinal level. Attachments and fiber directions of the posterior and anterior aspect of the facet joint capsule are also described,as well as how these fiber directions may relate to joint movements is discussed. The relationship of the tendons of the lumbar multifidus relative to the posterior joint capsule is described and its role in joint motion discussed. In addition, the attachments of the ligamentum flavum are described relative to the anterior aspect of the facet joint. Their role during joint motion and the sizes of the ligament are compared relative to gender, side and spinal level.
Materials and Methods
This descriptive anatomical study of bilateral lumbar joints L3-L4, L4- L5 and L5-S1 involved 13 male (78.6 + 11.3 y.o.) and 12 female (80.9 + 11.2 y.o.) cadavers. Male and female mean ages are not significantly different. Table 1 shows the number of facets studied at each level, along with the number and reason facets were not included. Cadavers were randomly obtained from the Anatomical Board of the State of Florida. Exclusion criteria were lumbar spine surgery, and scoliosis. The age and gender were recorded. A number was assigned to each study specimen, coinciding with the cadaver number and right or left side. This numbering system allowed specimen data to be matched directly to cadaver data for comparison and analysis. This study was approved and monitored by the Institutional Review Board of the University of St. Augustine for Health Sciences.
LEVEL
MALES (M)
FEMALES (F)
# WITH FUSED FACETS
L-LL3
23
24
0
R-LL3
23
24
0
L-UL4
23
24
0
R-UL4
23
24
0
L-LL4
24
23
1-M
2-F
R-LL4
24
24
1-M
1-F
L-UL5
25
23
0-M
2-F
L-UL5
24
24
1-M
1-F
L-LL5
25
22
0-M
3-F
R-LL5
24
23
1-M
2-F
L-S1
25
23
0-M
2-F
R-S1
24
22
1-M
3-F
L: Left; R: Right; LL: Lower lumbar; UL: Upper lumbar; S: Sacral
Table 1: Number of male and female specimens at each vertebral level sampled and the number of those facets excluded because of joint fusion.
Study specimens were obtained by removing and bisecting the lumbar spine from L3 to the sacrum. Vertebral removals early in the study resulted in damage to Lower Lumbar L3 (LL3) and Upper Lumbar L4 (UL4) and these were excluded from the study (Table 1). Each specimen was placed in a sealed plastic bag for storage. Muscles and other soft tissues were removed from each specimen, the articular joints were identified and the area cleaned, taking care not to damage the capsule. The soft tissues surrounding each joint were carefully removed using “fine” dissection tools. The tendons of the multifidithat were crossing the posterior joint capsule were drawn, the attachments recorded and then reflected. The capsules of each joint were studied using a lighted magnifying glass and an OMNI dissection scope with a ring illuminator with scope camera. For each of the joints studied, the capsule was sketched by the dissector until a consistent pattern arose for at least 20 joints at each level, and then verified in subsequent dissections. Toluidine blue stain was applied occasionally to help differentiate collagen fiber direction. The most common configuration of the joint capsule is described in the results.
After all the joints on one side of the bisected specimen were cleaned, the length, width and thickness of the ligamentum flava were measured. A total of 50 ligaments were measured; 7 male and 10 female from L3-L4 and L4-L5 and 6 male and 10 female from L5-S1. The vertebral disc and the ligamentum flavum at each level were then incised so that facet movements within the capsule and the facet interfaces were visible. The capsule was then carefully cut along the joint interface and the joint was dislocated. The collagen arrangements of the capsule were again examined and compared to the common capsular pattern with major variations noted. After each joint had been dislocated, the length and width of the facet were measured, using a caliper, under a 3 Doppler magnification lens with halo light and recorded.
Data Analysis
All statistical distribution and analysis was performed using version 21 of IBM SPSS Statistics (2010). Means ± 1SD for facet and ligament flava sizes and areas were calculated. Significances between age, gender, sidedness, size and vertebral level were determined using the Independent Samples Mann-Whitney U Test. The level of significance was p <.05.
Results
Each joint consists of an inferior facet from the vertebra above and the superior facet for the vertebra below. The inferior facets are medial/anteromedial to the superior facet. Between the facet articulations are small cephalad and caudal menisci (Figure 1). Many previous studies have described the boney anatomy of the facet joint and the orientation of the articular surfaces [1,6,12,16-18,20,21,44]. A brief description of facet shape is described below and the mean +1SD sizes of the lower L3 to the S1 facets are shown in Table 2. Comparisons of mean facet size, with age, gender and sidedness, show no significant differences (Figure 2 and 3).
Level
N
Range
Mean+ S.D.
LL3W
44
12 – 20
14.61+1.80
LL3H
44
13 – 23
17.27+2.43
LL3A
44
180 – 460
255.07+64.24
UL4W
44
14 - 21
16.66+1.84
UL4H
44
14 – 24
17.95+2.66
UL4A
44
198 – 480
302.68+72.16
LL4W
46
12 – 19
14.98+1.66
LL4H
46
14 – 22
17.22+1.78
LL4A
46
168 – 380
259.02+46.78
UL5W
46
14 – 24
17.46+2.34
UL5H
46
14 – 24
18.72+2.43
UL5A
46
225 – 529
329.09+75.08
LL5W
44
14 – 22
17.16+2.23
LL5H
44
14 – 22
18.84+2.50
LL5A
44
210 – 484
326.11+74.66
S1W
44
14 – 24
18.64+2.12
S1H
44
15 – 25
19.45+2.36
S1A
44
224 - 525
365.73+73.62
N : Number of specimens; A: Area; H: Height; LL: Lower lumbar; UL: Upper lumbar; S: Sacral; W: Width
Table 2: Overall sizes (mm) of the facets at each vertebral level. Notice the increase in areas for l5 and s1 compared to those for l3 and l4.
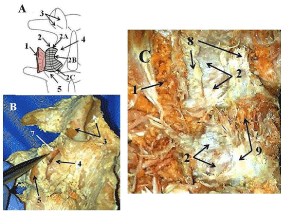
Figure 1: Posterior Facet Capsule and Multifidus: (A) Drawing of a lumbar
facet joint showing the capsule and attachment of the multifidus muscle. (B)
Photograph of a lumbar facet joint showing the cephaladmeniscus (forceps).
(C) Photograph of 2 lumbar facet joints showing the joint capsule and
multifidus muscle. 1. In (A), Multifigus muscle is cut and reflected laterally; in
(C) the multifidus is reflected medially and its tendon divided, 2. Joint capsule,
2A. Superior curved capsular fibers, 2B. Middle capsular fibers, 2C. Inferior
curved capsular fibers, 3. Superior articular process and facet, 4. Inferior
articular process, 5. Superior articular process, 6. Cephaladminicus held with
forceps, 7. Cut joint capsule, 8. Tendinous fascia from the multifidus muscle
covering the posterior joint capsule and 9. Muscle fiber and tendon of the
deep interlaminar layer of the multifidus.
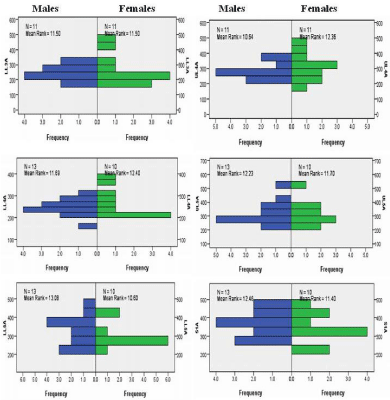
Figure 1: Independent-Samples Mann- Whitney U Test graphs of facet joint areas for gender. The top two graphs are for Lower Lumbar 3 (LL3, left) and Upper
Lumbar 4 (UL4, right). The middle two graphs are for Lower Lumbar 4 (LL4, left) and Upper Lumbar 5 (UL5, right). The bottom two graphs are for Lower Lumbar
5 (LL5, left) and Sacral 1 (S1, right).
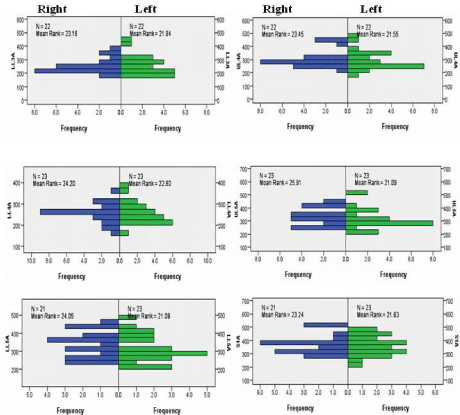
Figure 3: Independent-Samples Mann- Whitney U Test graphs of facet joint areas for side. The top two graphs are for Lower Lumbar 3 (LL3, left graph) and Upper
Lumbar 4 (UL4, right graph). The middle two graphs are for Lower Lumbar 4 (LL4, left graph) and Upper Lumbar 5 (UL5, right graph). The bottom two graphs are
for Lower Lumbar 5 (LL5, left graph) and Sacral 1 (S1, right graph).
The inferior facet of L3 is oval in shape with the superior and inferior poles slightly narrower than the middle. Its matching superior facet of L4 is quadrangular in shape and concave. The inferior facet of L4 is shaped like the inferior facet of L3, and the inferior facet of L5 is somewhat square in shape. The superior facets of L5 and S1 are quadrangular in shape and concave like that of the L4 superior facet. The inferior base of the L5 and the S1 superior articular process has a shallow bowl that extends to the inferior limit of the facet. This bowl is covered with articular cartilage and lies within the joint capsule.
The joint capsules of L3-L4, L4-L5 and L5-S1 show a similar collagen directional pattern. The posterior part of the joint capsule shows three directional bands of fibers (Figure 1). There are curved superior fibers, curved inferior fibers and middle horizontal running fibers between the two curved sets of fibers. The superior curved fibers form a distinct dome-like band that crosses the superior and superior-posterior parts of the joint. The curved superior fibers from the superior articular process arise from the superior part of a boney ridge on the posterior margin of the process. Superior fibers attaching to the inferior articular process attach at its superior-posterior margin. Deep to these curved superior fibers is a subcapsular pocket [1,19,22].
The middle horizontal fibers are continuous superiorly and inferiorly with the respective curving fibers of the capsule. These middle fibers generally run horizontally, but may also slant slightly downward from medial to lateral. Fibers from the superior articular process attach to the middle of the same posterior ridge described for the curved superior fibers. These fibers then run medially across the posterior- middle of the inferior process to attach at the junction of the inferior process and the lamina. This medial attachment of the middle fibers on the lamina produces a medialextension of the joint space (Figure 4).
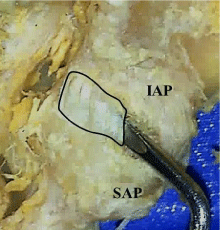
Figure 4: Medial extension of the facet joint capsule (outlined). IAP : Inferior
Articular Process, SAP : Superior Articular Process.
The posterior aspect of the joint capsule is covered with a tendinous fascia from the multifidus muscle (Figure 1). Because the multifidus originates medial to the joint, the dense fibers of its fascia run mainly horizontally across the joint with a slight superior-medial to inferior –lateral slant. These fibers cross over the inferior articular process, the facet joint articulation and posterior capsule, and attach to the posterior border of the superior articular process. From the vertically oriented deep interlaminar layer of the multifidus [37] (Figure 1), vertical running fibers attach to the lateral aspect of the joint capsule and posterior part of the superior articular process.
The anterior part of the joint capsule is a thin connective tissue sheet that is difficult to separate from the ligamentum flavum [18,25,47]. It appears to have superior and inferior parts. The superior part attaches to the superior- anterior margin of the inferior articular process and the anterior margin of the superior articular process. Its fibers slant downward from a medial to lateral direction. The inferior part covers the end of the inferior articular process and root of the superior articular process. The fibers of the inferior part run more vertically than those of the superior part but show a slight downward medial to lateral slant. This inferior part of the anterior capsule is attached to the adjacent surface of the ligament flavum (Figure 5 and 6).
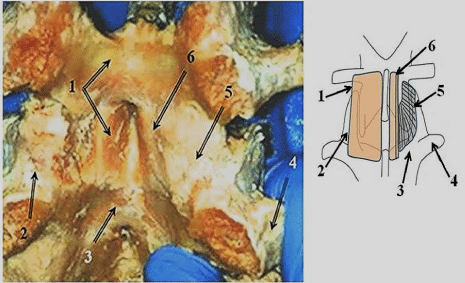
Figure 5: Anterior Capsule and Ligamentum Flavum: (Left) Photograph of
a dissection of the ligamentumflavum and anterior capsule. (Right) Drawing
of the same area. 1. Ligamentumflavum, 2. Superior articular process,
3. Lamina, 4. Transverse process, 5. Anterior facet joint capsule, 6. Cut
ligamentumflavum.
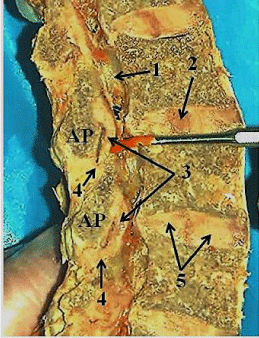
Figure 6: LigamentumFlavum: Photograph of the ligamentum flavum in
a saggital view in a preserved cadaver. The probe points to the saggitally
sectioned ligamentunflavum showing a space between it and the anterior
joint, the superior attachment of the ligament to the lamina and an inferior
attachment of the ligament to the joint capsule. 1. Spinal cord canal, 2.
Nucleus pulposus, 3. Ligamentumflavum, 4. Attachment of ligamentumflavum
to inferior joint capsule, 5. Annulus fibrous of intervertebral disc. AP : Articular
Process.
Covering the anterior aspect of the facet joint is the ligamentum flavum (Figure 5 and 6). The ligamentum flava observed in this study are very distinct and have similar dimensions. Table 3 shows the overall mean (+ 1 SD) width, height, thickness (middle) and area for the ligaments of L3-L4, L4-L5 and L5-S1 and Table 4 shows the mean relative to gender. There were no significant differences in size or ligament areas, or ligament areas for gender (Figure 7) and side.
Level
N
Mean+ S. D.
L3-4W
17
17.47+2.00
L3-4H
17
19.00+1.87
L3-4T
17
3.12+0.33
L3-4A
17
332.65+65.21
L4-5W
17
17.41+2.00
L4-5H
17
19.53+1.90
L4-5T
17
3.12+0.48
L4-5A
17
342.35+64.43
L5-S1W
16
18.13+2.33
L5-S1H
16
18.25+4.68
L5-S1T
16
3.06+0.25
L5-S1A
16
349.19+73.17
N: Number; L: Lumbar; S: Sacral; A: Area; H: Height; T: Thickness; W: Width
Table 3: Overall sizes (mm) of the Ligamentum Flava for vertebral levels L3-L4 to L5- S1.
Level
Gender
N
Mean+S. D.
L34W
1
7
17.00+2.30
2
10
17.80+1.81
L34H
1
7
18.86+2.26
2
10
19.10+1.66
L34A
1
7
319.57+79.09
2
10
341.80+56.25
L45W
1
7
16.43+1.98
2
10
18.10+1.79
L45H
1
7
19.14+2.79
2
10
19.80+1.03
L45A
1
7
317.57+79.50
2
10
359.70+48.53
L5S1W
1
6
17.33+2.58
2
10
18.60+2.17
L5S1H
1
6
18.67+2.16
2
10
19.80+1.47
L5S1A
1
6
313.67+78.49
2
10
370.50+64.46
1: Male, 2: Female; N: Number; L: Lumbar; S : Sacral; A: Area; H: Height; W: Width
Table 4: Sizes (mm) for gender of the Ligamentum Flava from L3-4 to L5-S1.
Each of these paired, rectangular, segmental, elastic ligaments is thin at its superior and inferior attachment sites but bulge centrally. The superior part of the ligament attaches to the inferior margin of the lamina of the vertebra above and to the inferior part of the superior margin of the lamina of the vertebra below. The superior – lateral part of the ligament attaches to the medial root of the inferior articular process and the inferior- lateral part to the upper anterior aspect of the superior articular process. The middle lateral margin of the ligament forms a free edge that crosses over the articulation site of the facets (Figure 6). Medially, each ligamentum flavum attaches to the medial limit of the lamina near the origin of the spinous process and the root of the interspinous ligament. The external surface of the ligamentum flavum is covered by a thin layer of fibrous tissue that is continuous vertically and medially with adjacent ligamentum flava.
Discussion
The lack of significant differences in the mean sizes and areas of the articular facets among levels, and between gender and sides, agrees with previous studies on the lumbar facet joints [4,5,12,44,48]. However, previous studies have found a significant difference between the facets and age [4,11-13,15,16]. In this study, the age range of the cadavers was small and so any significant age differences would be unexpected.
The specimens for this study were obtained randomly over a period of 3 years. Other than the cause of death and gender, no other medical history was available. While body size and weight differences may contribute to the size differences measured in this study, these were not recorded. Further, some of the differences in size may also relate to the embalming process. Because the exclusion criteria for this study were limited to only lumbar surgery and scoliosis, the specimens studied seem to provide data and conclusions that may relate to a more general population than studies in which the specimens were limited to a particular disease or disorder.
Comparing previous macroscopic and microscopic descriptions of the lumbar facet joint capsule [1,18,19,21-24,26], the capsule proper consists of fibers arranged horizontally, or with a slight medial to lateral downward slant. These descriptions agree with the middle fibers of the posterior capsule described here.However, the curving superior and inferior fibers of the capsule are described in only a few studies [1,25]. Facets joint attachments may also be classified as Type I or Type II dependent on the presence of a “subcapsular pocket” [1,19,22]. In this study, these pockets were common under the superior curving fibers and above the inferior curving fibers of the joint capsule.
As the facets move vertically during flexion and extension, these curved superior and inferior fibers and subcapsular pockets would allow vertical movements of the facets. Flexion would be limited by tension of the upper and lower curved fibers, but the superior fibers would undergo less tension during extension [1,42]. The inferior curving fibers would also close off the posterior aspect of the “bowl” lying at the base of the upper L5 and S1 facets and limit the extent of extension.
In addition, the medial posterior- inferior aspect of the L4 -L5 and L5 –S1 capsules showed an extension of the joint space. This extension was continuous with the joint space and large enough to easily insert a probe (Figure 4). Functionally, the posterior location of this extension would seem to increase posterior capsular space and allow increased distraction or gaping of the inferior facet during rotation and side bending, especially during trunk flexion. This stretching of the posterior capsule would be limited by the superior, middle and inferior parts on the capsule and the tendon of the multifidus.
The tendons of the multifidus muscle cover the posterior joint capsule. These tendons run in a medial to lateral direction, as well as vertically, producing an irregular, thick layer of connective tissue on the posterior aspect of the facet joint capsule. This tendinous layer attaches to the lateral margin of the posterior capsule in the area of the menisci, but it can be easily removed from the posterior surface of the capsule. While this irregular layer of tendinous connective tissue does reinforce the posterior capsule, it does not appear to be a part of the facet joint capsule. The site of attachment of the multifudus to the lateral posterior margin of the capsule supports the concept that the multfidus functions to keep the menisci and capsule from being entrapped between the moving facets.
Because the tendons of the multifidus attach loosely or not at all to the posterior surface of the capsule, these tendons would slide across the capsule and not directly restrict movements. However, tension on the crossing tendons by contraction of the multifidus would translate the ipsilateral inferior facet anterior-laterally and compress it against its matching ipsilateral superior facet. At the same time, the crossing tendons would tighten the posterior capsule to prevent posterior translation of the ipsilateral inferior facet. Anterior translation of the ipsilateral inferior facet would be restricted by the articular surface of the anterior facet, the anterior joint capsule and the ligamentum flavum. This point of compression between the ipsilateral inferior and superior facets is what forms a pivot point, allowing rotation of the vertebra to the opposite side. This compression may increase articular cartilage wear along the anterior aspect of the facet joint compared to its posterior aspect. This rotation to the contralateral side occurs as the contralateral inferior facet translates posterior-medially and distracts (gaps) away from the superior facet. The presence of the posteriormedial capsularextension and relaxation of the multifidus on the contralateral side would allow this posterior distraction motion.
On the anterior aspect of the facet joint are the thin anterior joint capsule and the ligamentum flavum. The vertical and downward medial orientation of the inferior fibers of theanterior capsular fibers would tend to limit the extent of inferior facet upward translation allowed by stretching of elastic ligamentum flavum. The anterior joint capsule and ligamentum flava may also limit anterior translation during opposite side rotation,as well as posterior translation andposterior distraction of the inferior facet relative to the superior facet.
Dissections of the ligamentum flavum agree with previous studies describing its macroscopic anatomy [1,18,32,33]. Direct attachments of adjacent ligaments were not observed, but a thin superficial sheet of connective tissue did cover the surface and extend vertically and medially between adjacent ligaments. Attachment of the ligamentum flava to the inferior part of the anterior capsule supports the concept that it minimizes the entrapment of the menisci and capsule between the facets during trunk motion. The size of the ligamentum flava and the lack of significance differences in the sizes among the lower lumbar vertebrae suggest it changes little in older adults. The thickness of the ligamentum flava in this study was also within the ranges described by others, using calipers on cadavers or MDIs on living subjects [27,30,33,49].
The lumbar facet joint complex is compromised in laminectomies, spinal fusion surgeries and degenerative disc disease. Complete removal of the spinal lamina damages the ligamentum flavum on the side of the surgery. Because this ligament provides anterior stability to the facet joint at that segment and is involved in segmental facet joint motion to match facet articular surfaces, its removal can produce subsequent post-surgical problems with joint instability and abnormal facet motion. These problems may result in increased loading and wear on the facet articular surface, promoting Facet Joint Osteoarthritis (FOA). The instability at the level of the surgery may also change the mechanics of segmental facet motion above and below the surgical site. While laminectomies can be accompanied by spinal fusion, the lack of segmental movements at the surgical site will also alter the segmental motion above and below. In both cases, future low back problems can occur. In addition, because of the removal of muscle from the lamina, in particular the lumbar multifidus, movements and support of the facet joints will be reduced at multiple spinal segments, decreasing back function, increasing facet joint wear and initiating low back pain.
With Degenerative Disc Disease (DDD), the approximation of the vertebral bodies increases the compression on the facet joints and changes the relative positon of the matching facet joint surfaces. The resultant increase in joint compression accelerates wear of the articular facet, producing osteoarthritic changes. The change in facet joint position will alter joint mechanics and motion, and may eventually change the collagen arrangement of the joint capsule. Because capsular connective tissue follows the principles of Wolf’s Law, portions of the capsule over time can shorten or elongate, and in areas of reduced tension, the capsule may become thin and predisposed to tearing. The insertion of disc replacements or the inflation of the disc space may damage the joint capsule because of changes in the arrangement and amount of collagen fibers. Any capsular damage may produce abnormal movements and stresses, resulting in advanced articular cartilage wear and FOA.
Conclusion
Movements at the lumbar facet joint involve the joint capsule, ligamentum flavum, multifidus muscle and articular surface. These components of the facet joint form a joint complex that allows, limits and stabilizes the joint. The anterior and posterior aspects of the joint capsule are different. During spinal flexion and extension, the different part of the posterior capsule stretch and the ligamantum flavum stretch and recoil and keeps the capsule from being pinched between the facets. During rotation, the multifidus anteriorly translates the inferior facet to compress the matching inferior and superior facets and produce a pivot joint which allows rotation to the opposite side.The anterior translation of the inferior facet is restricted by the anterior joint capsule and ligamementum flava. This compression during rotation may increase articular cartilage wear on the anterior aspect of the facet. On the side of rotation, posterior distraction of the inferior facet relative to the superior facet is permitted by relaxation of the multifidus and the posterior capsule and, perhaps, at L4 – L5, L5- S1 by the presence of a posterior medial extension of the joint space.
Acknowledgement
We thank Ms. Jackie Nelson and Dr. Erin Conrad for their very helpful comments and the University of St Augustine for Health Sciences for the time and resources to complete this study. We are also very grateful to all those who have donated their bodies to science so that studies such as this are possible.
References
- Bogduk N. Clinical and Radiological Anatomy of the Lumbar Spine and Sacrum. 5th edn. Elsevier, Churchill, Livingston. 2012.
- Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: the facet joints. Nature Reviews Rheumatology. 2013; 9: 216-224.
- Goode AP, Carey TS, Jordan JM. Low back pain and lumbar spine osteoarthritis: how are they related?. Current Rheumatology Reports. 2013; 15: 3051-3058.
- Jentzsch T, Geiger J, Zimmermann SM, Slankamenac K, Nguyen-Kim TDL, Werner CM. Lumbar Facet Joint Arthritis Is Associated with More Coronal Orientation of the Facet Joints at the Upper Lumbar Spine. Radiology Research and Practice. 2013; 2013: 1-9.
- Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: A review. Semin Arthritis Rheum. 2007; 37: 69-80.
- Kuo CS, Hu HT, Lin RM, Huang KY, Lin PC, Zhong ZC, et al. Biomechanical analysis of the lumbar spine on facet joint force and intradiscal pressure¯a finite element study. BMC MusculoskeletDisord. 2010; 11: 151.
- Laplante BL, DePalma MJ. Spine osteoarthritis. PM&R. 2012; 4: S28-S36.
- Suri P, Hunter DJ, Rainville J, Guermazi A, Katz JN. Presence and extent of severe facet joint osteoarthritis are associated with back pain in older adults. Osteoarthritis and Cartilage. 2013; 21: 1199-1206.
- Wei T, Yanwei L, Yajun L, Bin X, Xiao H. The High Prevalence of Symptomatic Degenerative Lumbar Osteoarthritis in Chinese Adults: A Population-Based Study. Spine. 2014; 39: 1301-1310.
- Manchikanti L, Pampati V, Falco FJ, Hirsch JA. Growth of spinal interventional pain management techniques: Analysis of utilization trends and medicare expenditures 2000 to 2008. Spine. 2013; 38: 157-168.
- Cubuk R, Kozakcioglu M, Tasali N, Celik L. Lumbar disc and facet degeneration: Correlation with age and facet orientation. TrakyaUniv Tip FakDerg. 2009; 26: 36-42.
- Kalichman L, Suri P, Guermazi A, Li L, Hunter DJ. Facet orientation and tropism: associations with facet joint osteoarthritis and degeneratives. Spine. 2009; 34: E579-E585.
- Otsuka Y, An HS, Ochia RS, Andersson GB, Orías AAE, Inoue N. In vivo measurement of lumbar facet joint area in asymptomatic and chronic low back pain subjects. Spine. 2010; 35: 924-928.
- Simon P, Espinoza Orías AA, Andersson GB, An HS, Inoue N. In vivo topographic analysis of lumbar facet joint space width distribution in healthy and symptomatic subjects. Spine. 2012; 37: 1058-1064.
- Taylor JR, Twomey LT. Age changes in lumbar zygapophyseal joints: Observtions on structure and function. Spine. 1986; 11: 739-745.
- Wang J, Yang X. Age-related changes in the orientation of lumbar facet joints. Spine. 2009; 34: E596-E598.
- Wilke HJ, Zanker D, Wolfram U. Internal morphology of human facet joints:comparing cervical and lumbar spine with regard to age, gender and the vertebral core. J of Anatomy. 2012; 220: 233-241.
- Behrsin JF, Briggs CA. Ligaments of the lumbar spine: A review. Surg. Radiol Anat.1988; 10: 211-219.
- Sato S, Oguma H, Murakami G, Noriyasu S. Morphological study of the joint surface and capsule of the lumbar zygapophysial joint with special reference to their laterality. Okajimas Folia AnatJpn. 2002; 79: 43-53.
- Twomey L, Taylor J. The lumbar spine: structure, function, age changes and physiotherapy. Australian journal of Physiotherapy. 1994; 40: 19-30.
- Varlotta GP, Lefkowitz TR, Schweitzer M, Errico TJ, Spivak J, Bendo JA, et al. The lumbar facet join a review of current knowledge: part 1: anatomy, biomechanics, and grading. Skeletal Radiology. 2001; 40: 13-23.
- Xu GL, Haughton VM, Yu S, Carrera GF. Normal variations of the lumbar facet joint capsules. Clin Anat. 1991; 4: 117-122.
- Xu GL, Haughton VM, Carrera GF. Lumbar facet joint capsule: appearance at MR imaging and CT. Radiology. 1990; 177: 415-420.
- Yahia LH, Garzon S. Structure of the capsular ligaments of the facet joints. Ann Anat. 1993; 175: 185-188.
- Yamashita T, Minaki Y, Ozaktay AC, Cavanaugh JM, King AI. A morphological study of the fibrous capsule of the human lumbar facet joint. Spine. 1996; 21: 538-543.
- Zhang Y, Yu L, Li YK. Clinical anatomy of the fibrous capsule of human lumbar facet joint. Journal of Bone and Joint Surgery. 2002; 22: 600-601.
- Abbas J, Hamoud K, Masharawi YM, May H, Hay O, Medlej B, et al. Ligamentum flavum thickness in normal and stenotic lumbar spines. Spine. 2010; 35: 1225-1230.
- Appolonio PR, Mattar T, Costa AB, ValesinFilho ES, Rodrigues LMR. Thickening of spine ligamentum flavum and facet tropism. Coluna/Columna. 2014; 13: 39-42.
- Haig AJ, Adewole A, Yamakawa KS, Kelemen B, Aagesen AL. The ligamentum flavum at L4-5: relationship with anthropomorphic factors and clinical findings in older persons with and without spinal disorders. PM&R. 2012; 4: 23-29.
- Sairyo K, Biyani A, Goel V, Leaman D, Booth R, Thomas J, et al. Pathomechanism of ligamentum flavum hypertrophy: a multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine. 2005; 30: 2649-2656.
- Spurling RG, Mayfield FH, Rogers JB. Hypertrophy of the ligamentaflava as a cause of low back pain. Journal of the American Medical Association. 1937; 109: 928-933.
- Tien Chau AM, Pelzer NR, Hampton J, Smith A, Seex KA, Stewart F, et al. Lateral Extent and Ventral Laminar Attachments of the Lumbar LigamentumFlavum: Cadaveric Study. Spine Journal. 2014; 14: 2467-2471.
- Yong-Hing K, Reilly J, Kirkaldy-Willis WH. The ligamentumflavum. Spine. 1976; 1: 226-234.
- Beresford ZM, Kendall RW, Willick SE. Lumbar facet syndromes. Current sports medicine Reports. 2010; 9: 50-56.
- De Foa JL, Forrest W, Biedermann HJ. Muscle fibre direction of longissimus, iliocostalis and multifidus: landmark-derived reference lines. Journal of Anatomy. 1989; 163: 243-247.
- Hesse B, Fröber R, Fischer MS, Schilling N. Functional differentiation of the human lumbar perivertebral musculature revisited by means of muscle fibre type composition. Annals of Anatomy AnatomischerAnzeiger. 2013; 195: 570-580.
- Lonneman E, Paris SV, Gorniak GC. A Morphological Comparison of the Lumbar Multifidus by Chemical Dissection. J Man & ManpTher. 2008; 16: 84-92.
- Macintosh JEVF, Bogduk N, Munro RR. The morphology of the human lumbar multifidus. Clinical Biomechanics (Bristol, Avon). 1986; 1: 196-204.
- Safak AA, Is M, Sevinc O, Barut C, Eryoruk N, Erdogmus B, et al. The thickness of the ligamentumflavum in relation to age and gender. Clinical Anatomy. 2010; 23: 79-83.
- Valencia F, Munro RR. Morphology of the lumbar multifidus in man. Journal of Anatomy. 1984; 139: 196.
- El-Bohy AA, Goldberg SJ, King AI. Measurement of facet capsular stretch. Proceeding to the Amer. Soc.of Mechanical Engineers, Bioengineering symposium New York. AmerSoc of Mechanical Engineers. 1987; 161-164.
- Hedtmann A, Steffen R, Methfessel J, Kolditz D, Kramer J, Thols M. Measurement of human lumbar spine ligaments during loaded and unloaded motion. Spine. 1989; 14: 175-185.
- Serhan HA, Varnavas G, Dooris AP, Patwardhan A, Tzermiadianos M. Biomechanics of the posterior lumbar articulating elements. Neurosurg Focus. 2007; 22: 1-6.
- Tischer T, Aktas T, Milz S, Putz RV. Detailed pathological changes of human lumbar facet joints L1-L5 in elderly individuals. Eur Spine J. 2006; 15: 308-315.
- Kozanek M, Wang S, Passias PG, Xia Q, Li G, Bono CM, et. al. Range of motion and orientation of the lumbar facet joints in vivo. Spine. 2009; 34: E689-E696.
- Ma HT, Griffin JF, Yang Z, Kwok AWL, Leung PC, Lee RYW. Kinematics of the lumbar spine in elderly subjects with decreased bone mineral density. Med &BiolEngin&Comput. 2009; 47: 783-789.
- Cyron BM, Hutton WC. The tensile strength of the capsular ligaments of the apophyseal joints. Journal of Anatomy.1981; 132: 145-150.
- Tanno I, Oguma H, Murakami G, Sato S, Yamashita T. Which portion in a facet is specifically affected by articular cartilage degeneration with aging in the human lumbar zygapophysial joint?. Okajimas Folia AnatJpn. 2003; 80: 29-34.
- Fukuyama S, Nakamura T, Ikeda T, Takagi K. The effect of mechanical stress on hypertrophy of the lumbarligamentumflavum. Journal of Spinal Disorders & Techniques. 1995; 8: 126-130.