
Research Article
Austin J Anat. 2015;2(2): 1036.
Regional Variation in the Histomorphology and Tensile Strength of the Ventral Rectus Sheath in the Male Goat (Capra Hircus)
Odula PO¹*, Hassanali J¹ and Kiama SG²
¹Department of Human Anatomy, University of Nairobi, Kenya
²Department of Veterinary Anatomy and Physiology, University of Nairobi, Kenya
*Corresponding author: Odula PO, Department of Human Anatomy, University of Nairobi, P.O. Box 25540- 00603 Nairobi, Kenya
Received: May 18, 2015; Accepted: July 14, 2015; Published: July 18, 2015
Abstract
The ventral rectus sheath (VRS) plays a key role in the stabilization of the ventral abdominal wall. This sheath has to be particularly strong in ruminants to accommodate the viscera and the large quantities of forage in their stomach. This study was conducted to establish the structural and the mechanical properties of the ventral rectus sheath in the goat, a browser, in order to elucidate its function. The ventral rectus sheath was formed by superficial and deep lamina consisting of obliquely aligned collagen fibers derived from the external and internal oblique abdominal aponeuroses respectively. Closely apposed and intimately held to the superficial lamina was a layer of longitudinally aligned elastic fibers, the ttunica flava abdominis or modified deep fascia. This tunica flava abdominis progressively increased in thickness from the epigastrium to the hypogastrium. On tensiometry, the epigastric ventral rectus sheath withstood about half the load (50N/mm2) required to reach yield point compared to the umbilical ventral rectus sheath (94.5N/mm2). Furthermore, the Youngs modulus showed that the umbilical ventral rectus sheath was the stiffest at 669 (SD 22.2) N/mm2 while the epigastric ventral rectus sheath was the weakest at 554 (SD 29) N/mm2 respectively when exposed to longitudinal traction. In conclusion, the progressively thickening of the tunica flava abdominis and the superficial lamina from the epigastrium to the hypogastrium may confer reversible stretch ability and strength to the ventral rectus sheath and is therefore well suited for longitudinal load strength needed to support the compound stomach during browzing.
Keywords: Goat; Ventral rectus sheath; Collagen fibers; Tunica flava abdominis; Elastic fibers; Tensile strength
Abbreviations
LA: Linea Alba; EM: External Oblique Abdominis Muscle; RM: Rectus Abdominis Muscle; VRS: Ventral Rectus Sheath; EVRS: Epigastric Ventral Rectus Sheath; UVRS: Umbilical Ventral Rectus Sheath; HVRS: Hypogastric Ventral Rectus Sheath; PS: Pubic Symphysis; XP: Xiphoid Process; DF: Deep Fascia; SL: Superficial Lamina; DL: Deep Lamina
Introduction
The ventral abdominal wall is formed by the aponeurotic tendon of the abdominal wall muscles. The aponeuroses wrap the rectus abdominis muscle before forming the linea alba ventrally. The aponeuroses wrapping the rectus abdominis muscle ventrally is termed the ventral rectus sheath. It plays a key role in the stabilization of the ventral abdominal wall [1]. This tough tendinous structure is formed by the interlocking aponeurotic fibers of the external oblique abdominis and the superficial lamellae of the internal oblique abdominis [2,3]. In herbivores, the ventral rectus sheath is reinforced by the tunica flava abdominis which is part of the deep fascia of the trunk [4].
Ruminants like the goat, have a stomach capacity which is capable of accommodating large quantities of forages causing marked abdominal distension. Furthermore, goats have the ability to stand on their hind legs for long periods and sometimes can even climb in order to reach parts of trees they prefer for browsing [5]. Studies on the human ventral rectus sheath provided insights into its biomechanical structure [1,6]. However, apart from the macroscopic work done by Walmsey, the structure of the ventral rectus sheath of the goat is largely unknown [2]. This is despite the fact that the ventral abdominal hernias are fairly common in goats [7,8]. For instance, retrospective studies by Abdin-Bey and Ramadan reported a prevalence rate of ventral abdominal hernias of more than to 88% in adult goats who presented with hernia swellings [9].
The aim of this study is to describe the histomorphology of the ventral rectus sheath and evaluate its tensile strength in the goat. This may explain how the ventral rectus sheath of the goat, as a quadruped grazer and browser, is adapted to its function.
Materials and Methods
The ventral abdominal wall was harvested from 1- 2year old 6 (six) healthy male goats (Capra hircus) weight range 10-13kg. The goats were procured from a local butchery immediately after slaughter.
The skin and fascia were incised along a midline abdominal incision to form skin flaps that exposed the anterior abdominal wall aponeuroses. The xiphoid process, the umbilicus, and the pubic tubercle were used as landmarks. Using a metal template (40mm x 20mm), pieces of the ventral rectus sheath were resected from the mid-epigastric (EVRS), the umbilical (UVRS) and mid- hypogastric (HVRS) (Figure 1).
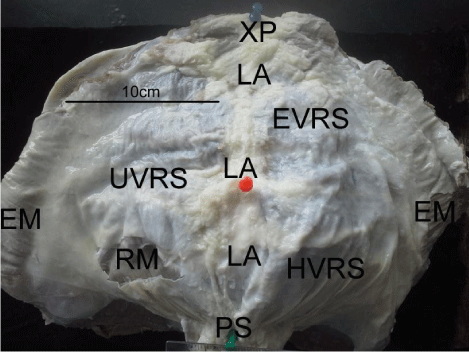
Figure 1: A picture of the ventral abdominal wall of a 2-year old male goat
showing the resection areas from the ventral rectus sheath and other relevant
landmarks.
Abbreviations: LA: Linea Alba; EM: External Oblique Muscle; RM: Rectus
Abdominis Muscle; EVRS: Epigastric Ventral Rectus Sheath; UVRS:
Umbilical Ventral Rectus Sheath; HVRS: Hypogastric Ventral Rectus Sheath;
PS/ green pin: Pubic Symphysis; Red pin: Umbilicus; XP/ blue pin: Xiphoid
Process
Light microscopy
A transverse section (20mm x 10mm) was fixed in 10% formalsaline. Thereafter, they were trimmed and dehydrated through increasing concentrations of alcohol from 50% to 100%, cleared with cedar wood oil and then embedded in paraffin wax. Seven micrometer (7um) sections were cut from paraffin blocks using a LEITZ WETZLAR sledge microtome (LeicaR Model SM2400, Leica Microsystems, Nussloch GmbH, Germany), collected on slides, stained with the Weigert resorcin–fuchsin stain and counterstained with Van Gieson stain for demonstration of elastic fibers [10]. Fifty six sections from each region were selected for histomorphometry. They were examined under a Leica Light microscope (BME model, Germany) at a various magnifications. Photomicrographs were taken using ZeissTM digital photomicroscope (Carl Zeiss AG, Oborkochen, Germany) and images analyzed with Image J software [11].The different layers of each region of the ventral rectus sheath thickness were measured in millimeters and the ratios plotted on a graph.
Tensiometry
The present study used a protocol employed by Seifert et al [12]. A Hounsfield tensiometer (80035; Pesola, Baar, AG Switzerland) was used to test the mechanical properties of the skin of the African spiny mice. Fresh strips of 30mm x 20mm, excised from the epigastric, umbilical and hypogastric parts of the ventral rectus sheath from 6 different goats, were kept in phosphate buffered saline before mechanical testing.
The strips were placed firmly in metal screw clamps with rubber pieces covering the clamp ends of the tensiometer, to be stretched automatically at a rate of 20mm/min. Each test took on average 2 to 4 minutes to complete. Tissue width (mm) and thickness (mm) at the waist (middle third of the tissue between the clamps) were measured using a vernier caliper before loading was started. Force (N) and displacement (mm) were measured on a xy plotter, and these points were subsequently recorded as stress (force per cross sectional area) and strain (fractional change in length) and repotted in Microsoft Excel (Microsoft Office Professional 2013). Longitudinal load was applied to the strips and the tensile strength and failure strain were recorded at the yield point.
When a sample tore or ruptured outside the measured area (outside the waist), the result was excluded from the analysis. The mean stress strain curves were then plotted in a graph to compare the three regions examined. No prior preconditioning was done on the tissues.
Results
Microscopy
The VRS of the three regions contained collagen and elastic fibers which were organized into two laminae namely, a superficial and a deep one in relation to the subcutaneous tissue. In all the three regions, the superficial lamina and the deep lamina consisted of rows of obliquely aligned collagen fibers (Figures: 2A - 2C).
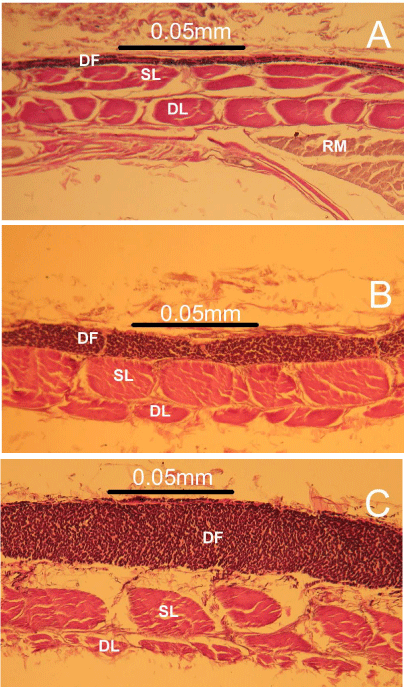
Figure 2: Photomicrographs of transverse sections of the epigastric (A),
umbilical (B) and hypogastric (C) ventral rectus sheath of the goat showing
the characteristic zones. Note the prominent black, elastic fibers found in the
deep fascia (DF) and the oblique collagen bundles found in the superficial
(SL) and deep laminae (DL). The thick zone of longitudinally aligned black
elastic fibers progressively increases in size from (A) to (C).
Abbreviations: DF: Deep Fascia; SL: Superficial Lamina; DL: Deep Lamina
Intimately adherent to the superficial laminae were rows of longitudinally aligned elastic fibers which made up the deep fascia or tunica flava abdominis (Figures: 2A- 2C). The collagen bundles in the deep lamina reduced markedly size relatively in a craniocaudal direction in contrast to the zone of rows of longitudinally aligned elastic fibers seen in the deep fascia, which became more prominent and thicker in size caudally, towards the hypogastric VRS (Figures: 2A - 2C).
In terms of actual size, the deep lamina was the thinnest at an average of 0.007mm (p-0.008) in the hypogastric VRS. The superficial lamina and the deep fascia on the other hand were thickest at 0.032mm (p-0.002) and 0.037mm (p-0.007) in the umbilical and hypogastric VRS respectively. The deep fascia was nearly 50% of the wall section in the hypogastric VRS compared to less than 20% in the epigastric VRS (Figure 3). The superficial lamina nearly doubled in size from 0.016mm in the epigastric VRS to 0.032mm in the umbilical VRS.
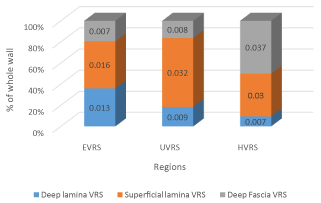
Figure 3: A bar chart showing percentage proportions mean measurements
of the different zones of the goat ventral rectus sheath at the different
regions. Note how the deep lamina gradually reduced in size proportionately
craniocaudally, compared to the superficial lamina and the deep fascia which
in contrast increased in size relatively. (N.B the measurements inserted within
the bars are in millimeters.)
Abbreviations: VRS: Ventral Rectus Sheath; EVRS: Epigastric Ventral
Rectus Sheath; UVRS: Umbilical Ventral Rectus Sheath; HVRS: Hypogastric
Ventral Rectus Sheath
Tensiometry
During mechanical loading, the VRS displayed elastic properties before breaking at its yield point. The greatest elasticity/ strain encountered, when exposed to a mean longitudinal load, was seen in the umbilical VRS. The yield point for the umbilical VRS was 94.5N/ mm2 at a strain of 0.23, the hypo gastric VRS was 76.8N/mm2 at a strain of 0.2 while the epigastric VRS was 50N/mm2 at a strain of 0.22 (Figure 4) respectively.
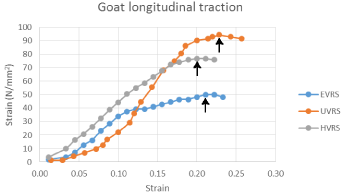
Figure 4: A graph showing the longitudinal stress/ strain curves on the
various parts of the goat ventral rectus sheath with the arrows pointing to the
yield point of each region of the ventral rectus sheath.
Abbreviations: EVRS: Epigastric Ventral Rectus Sheath; UVRS: Umbilical
Ventral Rectus Sheath; HVRS: Hypogastric Ventral Rectus Sheath
The ventral rectus sheath’s Youngs modulus was calculated over linear portions of the stress strain curves. These values show that on average the umbilical ventral rectus sheath, which was the stiffest at 669 N/mm2, was nearly 20% stiffer than the epigastric ventral rectus sheath when exposed to longitudinal traction (Figure 5).
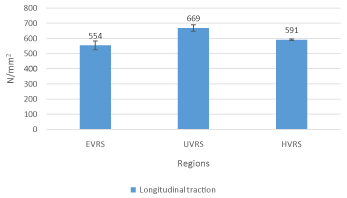
Figure 5: A bar chart showing Young’s modulus of the different regions of the
goat ventral rectus sheath when it was exposed to longitudinal traction. Note
how the umbilical ventral rectus sheath recorded the greatest stiffness at
669N/mm2 compared to the hypogastric ventral rectus sheath and epigastric
ventral rectus sheath.
Abbreviations: VRS: Ventral Rectus Sheath; EVRS: Epigastric Ventral
Rectus Sheath; UVRS: Umbilical Ventral Rectus Sheath; HVRS: Hypogastric
Ventral Rectus Sheath
Discussion
Observations of the present study showed that the ventral rectus sheath comprises of two collagenous laminae and an intimately adherent superficial elastic zone. The elastic zone, which forms the tunica flava abdominis, increases five times in thickness from the epigastrium to the hypogastrium. There is a scarcity of literature regarding the cytoarchitecture of the rectus sheath in the ruminants. However, in work done on the human rectus sheath by Mwachaka et al. [6] all the layers observed were made of predominantly collagen fibers. The prominent unique admixture of obliquely aligned collagen and longitudinal elastic fibers observed in the VRS of the goat may constitute a “strong aponeurosis of variable tension” [13,14]. The collagen fibers provide tensile strength [15] while elastic fibers allow stretch and recoil of tissues to their original shapes and sizes [16]. Mc Ardle proposed that the fibro elastic composition of the rectus sheath may be a design to enable it to withstand high levels of biomechanical stresses emanating from the pull of the muscles and also to tolerate sudden changes in the intra-abdominal pressure [17].
The elastic fibers in this study exhibited cranio-caudal asymmetry. By virtue of its position, the ventral rectus sheath is subjected to multidirectional forces emanating from the pull of muscles and from the stretching of the skin overlying it [17]. The high content of elastic fibers seen may help the reinforce the VRS to withstand the weight of the viscera and provide a possibility for stretching during browsing when the weight is likely to shift more toward the umbilical and hypo gastric region. Accordingly, it is suited to accommodate the rapid forces associated with strenuous activity which might otherwise cause injury to the abdominal viscera [18] or lead to increased incidences of hernia.
The regional variation described in the present study may explain the anatomical basis for the findings for biomechanical studies done by others [19]. Rath et al. using a dynamometer and a “bursting strength tester” demonstrated that the sub umbilical (infraumbilical) region of the human LA exhibits a larger coefficient of biomechanical elasticity than the supraumbilical region [19]. The apparent doubling of the collagenous superficial lamina from the epigastric VRS to the umbilical VRS seen in the current study represents the remarkable strength of aponeurotic fibers of the external oblique abdominis. This supports the abdomen during browsing and together with the elastic fibers in the deep fascia allows uniform distribution of forces generated [20]. When functioning together, these fibers make the VRS in the goat both compliant and resistant to mechanical stretch respectively.
The present study demonstrated that the epigastric ventral rectus sheath of the goat had the least stiffness compared to the umbilical ventral rectus sheath and the hypogastric ventral rectus sheath when exposed to longitudinal traction. Sheep and goats frequently present with different forms of hernias which may occur when the abdominal wall is severely traumatized, and these hernias may be high or low in the flank [21]. Due to the low yield point noted in this study, any violent force or overstretching of the abdominal muscles may easily tear the epigastric ventral rectus sheath of the goat and hence predispose it to herniation.
In conclusion, the progressively thickening of the elastic tunica flava abdominis from the epigastrium to the hypogastrium may confer reversible stretch ability to the ventral rectus sheath. The collagenous superficial lamina makes the umbilical and hypogastric ventral rectus sheath well suited for longitudinal load strength to support the compound stomach when browzing.
Acknowledgement
We thank John Kahiro and David Macharia for assisting during material testing and the department of Mechanical Engineering, University of Nairobi, for use of their equipment. We would also like to appreciate the efforts of Stephen Muita, Sarah Mungania and Acleus Murunga for assistance with animal care and materials procurement, Margaret Irungu for help with labeling and histological processing of the tissues for examination. Funding for this research was provided for by a Deans’ Committee grant from the University of Nairobi.
References
- Axer H, Keyserlingk DG, Prescher A. Collagen Fibers in Linea Alba and Rectus Sheaths I. General scheme and morphological aspects. J Surg Res. 2001a; 96: 127-134.
- Walmsley R. The Sheath of the Rectus Abdominis. J Anat. 1937; 71: 404-414.
- Askar OM. Surgical anatomy of the aponeurotic expansions of the anterior abdominal wall. Ann R Coll Surg Engl. 1977; 59: 313-321.
- Klaus-Dieter B. Bovine anatomy: An illustrated text, 2nd edition (Vet (Schlutersche)). 2003; 66.
- Yami A, Merkel RC. Sheep and goat production handbook for Ethiopia. Produced by Ethiopia sheep and goat productivity improvement program (ESGPIP). Funded by USAID. 2013.
- Mwachaka PM, Odula PO, Awori KO, Kaisha WO. Regional variations in the microscopic organization of the human rectus sheath. Journal of Morphological Sciences. 2009; 26: 84-90.
- Al-Sobayil FA, Ahmed AF. Surgical treatment for different forms of hernias in sheep and goats. J Vet Sci. 2007; 8: 185-191.
- Vilar JM, Corber JA, Spinella G. Double-layer mesh hernioplasty for repairing umbilical hernias in 10 goats. Turkish Journal of Veterinary and Animal Sciences. 2011; 35: 131-135.
- Abdin-Bey MR, Ramadan RO. Retrospective study of hernias in goats. Scientific Journal of King Faisal University, Saudi Arabia. 2001; 2: 77-86.
- Drury RAB, Wallington EA, Cameron R. Connective Tissue Fibers. Carleton’s histological techniques. 4th edition. New York. Oxford university press. 1967; 166-181.
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9: 671-675.
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature. 2012; 489: 561-565.
- Axer H, Keyserlingk DG, Prescher A. Collagen Fibers in Linea Alba and Rectus Sheaths II. Variability and Biomechanical Aspects. J Surg Res. 2001b; 96: 239-245.
- Fitzgibbons RJ, Greenburg AG, Nyhus LM. Nyhus and Condon’s Hernia. 5th edition. Lippincott Williams and Wilkins. 2002; 401.
- Parry DA, Craig AS, Barnes GR. Tendon and ligament from the horse: an ultrastructural study of collagen fibrils and elastic fibres as a function of age. Proc R Soc Lond B Biol Sci. 1978; 203: 293-303.
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002; 115: 2817-2828.
- McArdle G. Is inguinal hernia a defect in human evolution and would this insight improve concepts for methods of surgical repair? Clin Anat. 1997; 10: 47-55.
- Kirker-Head CA, Kerwin PJ, Steckel RR, Rubin CT. The in vivo biodynamic properties of the intact equine linea alba. Equine Vet J Suppl. 1989; 98-106.
- Rath AM, Attali P, Dumas JL, Goldlust D, Zhang J, Chevrel JP. The abdominal linea alba: an anatomo-radiologic and biomechanical study. Surg Radiol Anat. 1996; 18: 281-288.
- Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol. 2002; 65: 109-126.
- Tirgari M. Ventral hernia in the sheep. Vet Rec. 1980; 106: 7-9.