
Research Article
Austin J Anat. 2017; 4(2): 1065.
Effect of Ginseng on the Testis of Subclinical Hypothyroidism Model in Adult Male Albino Rat
Issa NM and El-Sherif NM*
Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Egypt
*Corresponding author: El-Sherif NM, Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Egypt
Received: January 31, 2017; Accepted: February 13, 2017; Published: February 17, 2017
Abstract
Hyperstimulation of follicular cells using a goitrogen Propylthiouracil (PTU) caused Subclinical Hypothyroidism (SCH) that was associated with disturbances in adult male testicular functions. This work was carried out to study the probable relationship between follicular cells in drug-induced hypothyroidism caused by PTU and consequently its effect on testicular tissue and the possible modulating effect of ginseng. The present work was carried out on 40 adult male albino rats divided into four groups. Group I was the control group; Group II (Ginseng treated); Group III (SCH group) rats and Group IV was the treated group. Testes and thyroid glands were studied. Our results revealed the protective role of Ginseng on testis and thyroid tissue of experimentally induced hypothyroidism rat model.
Keywords: Subclinical hypothyroidism; Follicular cells; Testis
Introduction
Thyroid hormone plays an important role in testicular development and function. It has been established that Triiodothyronine (T3) regulates the maturation and growth of the testis, controlling Sertoli cells and Leydig cell proliferation and differentiation during testicular development in rats and other species [1]. Subclinical Hypothyroidism (SCH) could be considered as an elevated serum thyrotropic stimulating hormone (TSH) level associated with normal Thyroxine (T4) and Triiodothyronine (T3) levels with no clinical symptoms of hypothyroidism. This thyroid dysfunction occurs in 4-20% of the adult population, the possibility of SCH progression to clinical hypothyroidism is around 7% [2]. Hypothyroidism extremely affects reproductive functions including fertility, pregnancy and postnatal development in human and rat [3]. Rat SCH model was induced mostly by administration of antithyroid drug mostly Propylthiouracil (PTU) and Methimazole (MMI) [4]. PTU is a thiouracil-derived drug which can be used to treat hyperthyroidism through lowering the amount of thyroid hormone produced by the thyroid gland [5]. PTU inhibits conversion of T4 to T3 causing hypothyroidism [6]. Ginseng is considered as an aromatic herb that is commonly used in herbal medicine [7]. It contains saponins and high iodine content [8]. Ginseng saponins, which are known as ginsenosides, have been studied to be responsible for its medicinal effects, specifically anticancer, antihypertensive, antidiabetic, and antistress effects. Ginseng benefits all the endocrine system, and therefore the thyroid gland. As ginseng markedly increased epididymal sperm count, motility and hyperactivation in mice [9]. So, we studied its effect on the testis and thyroid of SCH model.
Methodology
Animals
Forty adult Westar male albino rats were used in this experiment, each weighting 150-200gm. Food and water were provided ad libitum and the rats were left for 7 days for acclimatization before use in the Anatomy Department, Faculty of medicine, Menoufia University. All aspects of animal care and treatment were carried out according to the local guidelines of the ethical committee for animal research.
Experimental plan
The animals were divided into four groups as the following:
Group I (Control): included ten rats were kept without any treatment throughout the experimental period.
Group II (Ginseng treated): included ten rats that received ginseng (Panax ginseng) a product of Pharco Pharmaceuticals, Alexandria, Egypt, was available in the form of capsules with the trade name ‘Ginseng 100’. Each capsule contained 100mg of the dried roots of Panax ginseng. The contents of the capsule was withdrawn by a syringe and dissolved in distilled water and given to the rats in a dose of 2mg/kg once daily for three weeks by gastric tube.
Group III (Subclinical hypothyrid SCH group): included ten rats that received oral propylthiouracil in a dose of 5mg/kg once daily for three weeks by gastric tube.
Group IV (Treated group): included ten rats with subclinical hypothyroidism that received oral treatment of ginseng in a dose of 2mg/kg dissolved in distilled water once daily for three weeks by gastric tube two weeks after the induction of subclinical hypothyroidism.
Hormonal assay
Two weeks after subclinical hypothyroidism induction, retroorbital blood samples of all rats were collected and plasma Thyrotropic Stimulating Hormone (TSH), Plasma Thyroxine (T4) and Plasma triiodothyronine (T3) were measured. Sera were separated and stored at −20°C until the hormonal assay was performed. Serum levels of T3 and T4 were determined by chemilumines-cence immunoassay using Amerlite kits (Bunkyo-ku, Tokyo, Japan) [10]. Serum levels of TSH were assayed by radioimmunoassay [11]. Serum levels were measured at the Clinical pathology Department, Faculty of Medicine, Menoufia University.
Epididymal spermatozoal study
Twenty-four hours after the last treatment, animals were euthanized under anesthesia, according to the time of the experiment. The epididymal content of each rat was obtained immediately by cutting the tail of the epididymis and squeezing it gently to obtain fresh undiluted semen in a clean Petri dish to proceed for epididymal spermatozoal examination.
Histological study
Thereafter, animals were killed by cervical dislocation under ether anesthesia. The testes and thyroid gland of each rat were immediately excised and prepared for histological study. The right testis and the right thyroid lobe was cut into small pieces (about 1×1mm3) and fixed in 3% phosphate-buffered glutaraldehyde and was further processed and prepared for transmission electron microscopic examination [12] using a Joel 100 CX Electron Microscope (Joel, Tokyo, Japan) at the Electron Microscopy Unit, Faculty of Science, Menoufia University. Left testis samples were fixed in bouin’s solution while left thyroid samples were fixed in 10% formol saline and processed to prepare 5μm-thick paraffin sections for:
- Haematoxylin and Eosin (H&E) stain.
- Immunohistochemical stains for testis and thyroid sections.
Testicular sections were incubated with rabbit polyclonal anti- Androgen Receptors (anti-AR) antibody. Fremont, California, USA (Thermo Scientific) [13] while thyroid sections were incubated with rabbit monoclonal Anti-Thyroglobulin antibody. Abcam’s RabMAb technology, USA.
Quantitative assessment
The following parameters were measured:
- Area % of immunopositive reaction of anti-AR antibody.
- Area % of immunopositive reaction of Anti-Thyroglobulin antibody.
Five non-overlapping fixed fields, each 2mm2 in area, were taken using a Leica DMLB2/11888111 microscope equipped with a Leica DFC450 camera using the Leica C PLAN 10×0.22 objectives.
Statistical analysis
Data from the hormonal assay and immunohistochemical study were tabulated and expressed as mean±SD. They were fed into the computer using statistical package for the social sciences (SPSS, version 20; IBM SPSS, Chicago, Illinois, USA) software package [14]. Statistical analysis was carried out using one-way analysis of variance and the post-hoc test (Scheffe) for pair wise comparison. A level of significance of p<0.05 was considered to be statistically significant.
Results
There was no significant difference between Group I (Control) and Group II (Ginseng treated) rats in all the outcomes; therefore, these two groups were pooled in one group (Control).
Hormonal assay
The biochemical profiles of the three groups are shown in (Table 1). SCH was confirmed by measuring plasma T3, T4 and TSH levels. The serum TSH concentration in the SCH group was significantly increased compared with that in the control group.
Parameters
Control
Mean±SD
SCH
Treated
T3 (ng/ml)
13.83±0.19
T4 (μg/dl)
13.22±9.56
3.95±0.48
87±0.15
12.81±10.3
77.4±6.41
9.45±0.12*
72.62±9.43
TSH (μlU/ml)
2.83±0.290
*Significant difference (P<0.05) as compared with the control group.
0Significant difference (P<0.05) as compared with the SCH group.
Table 1: Serum thyroid hormone, TSH levels.
Epididymal spermatozoal study
Results showed a statistically significant decrease (P<0.05) in the mean sperm count, viability and motility when compared with the control group (Table 2).
Parameters
Mean±SD
Control
SCH
Treated
Sperm count
72.68±1.8
39.78±3.74*
66.32±0.120
Sperm viability (%)
91.37±4.34
39.22±2.57*
82.76±1.130
Sperm motility (%)
84±0.65
39±5.22*
75.98±7.880
*Significant difference (P<0.05) as compared with the control group.
0Significant difference (P<0.05) as compared with the SCH group.
Table 2: Effects of SCH on rat testes.
Histological study
Haematoxylin and Eosin (H&E) stain for testis sections: In the control group, testes exhibited normal histological patterns of the seminiferous tubules with normal shape and arrangement of their cellular differentiation. Spermatogonia and Sertoli cells rest on intact basement membranes. Large primary spermatocytes with characteristic large rounded nuclei, round spermatids, and elongated spermatids were observed. Spermatozoa were also visible in the lumen (Figure 1A and 1B).
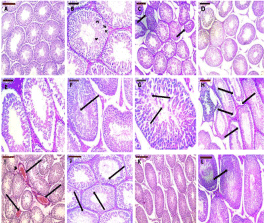
Figure 1: Representative H&E staining of rat testes of different experimental
groups. The testes of control rats showed normal organization of the
seminiferous tubules with narrow interstitial spaces (A). Seminiferous
tubules of testes of control rat exhibited normal spermatogenesis with
normal arrangement of Spermatogonia (Sg) and Sertoli Cells with triangular
nuclei (SC) resting on an intact basement membrane. Typical structures of
Primary Spermatocytes (PS), round and elongated Spermatids (SP) were
found (B). In the testes of SCH group, some seminiferous tubules showed
irregular boundaries (arrows, C), and many exhibited maturation arrest with
no mature spermatozoa in their lumen (D and E), sloughed germinal epithelial
cells (arrow, F), multiple giant cell formation (arrows, G). In some tubules,
the germinal epithelium was separated from the basement membrane
(arrows, H). The interstitium showed acidophilic hyaline material and dilated
congested blood vessels (arrow head, arrows, I). Many spermatocytes
appeared with pyknotic nuclei and surrounded by clear halos (arrows,
J).Testis sections of the treated rats showed significant improvement of
histological architecture with almost normal organized seminiferous tubules
with normal spermatogenesis (K and L); however, a few seminiferous tubules
showed pyknotic nuclei (arrow, K). Scale bar = 200 μm (A, C, D, H, I, and K)
and 100 μm (B, E-G, J, and L).
In SCH group, several seminiferous tubules showed irregular outlines and the majority showed maturation arrest. Germinal epithelial cells were sloughed in the lumina of some seminiferous tubules. In addition, many seminiferous tubules exhibited multiple giant cell formation. Many of the spermatocytes appeared with pyknotic nuclei surrounded by a halo indicating apoptosis. The interstitial connective tissue showed acidophilic hyaline material and dilated congested blood vessels (Figure 1C-J).
Treatment of rats with ginseng almost completely ameliorated the histopathological changes; however, a few of the seminiferous tubules still exhibited pyknotic nuclei (Figure 1K and L).
Immunohistochemical stain for testis sections: The SCH group dramatically down-regulated the expression of Androgen receptors as compared with control group. Ginseng co-administration significantly increased the expression of Androgen receptors (Figure 2).
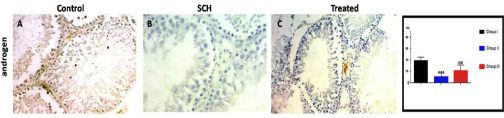
Figure 2: Expression of androgen receptors of experimental groups. Ginseng significantly up-regulated the SCH induced decrease in expression of Androgen
receptors. ***p<0.001, compared to the control group; and 000p < 0.05 compared to the SCH group. Scale bar = 100μm.
Electron microscopic examination of the testis: Ultrastructurally, control group showed that the seminiferous tubules were surrounded by basement membranes ensheathed by myoid cells. They were lined by spermatogenic and Sertoli cells. Spermatogonia appeared with peripheral heterochromatin in their nuclei and had a few mitochondria. Sertoli cells showed triangular indented euchromatic nuclei, prominent nucleoli; dense bodies, mitochondria were observed (Figure 3). Primary spermatocytes were distinguished by their large rounded nuclei. Spermatocytes showed thin rim of cytoplasm containing mitochondria that appeared small and peripheral (Figure 4). Spermatids appeared with large rounded euchromatic nuclei. Their cytoplasm appeared granular and showed peripherally located vesicular mitochondria (Figure 5). Interstitial Leydig cells were observed. These cells were relatively large in size with slightly elongated indented nuclei with a rim of peripheral heterochromatin. Their cytoplasm showed multiple processes (Figure 6). Transverse sections in different parts of the sperms revealed mid and principal pieces with a central axoneme. In the mid pieces, this axoneme was surrounded by dense bundles of fibrous sheath and mitochondrial sheath. In the principal pieces, the axoneme was surrounded by a fibrous sheath only (Figure 7).
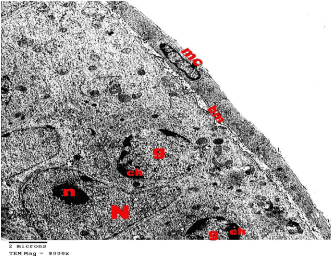
Figure 1: Electron microscopy of the testis of control group showing a part
of a seminiferous tubule surrounded by regular basement membrane (bm)
ensheathed by myoid cells (mc). Spermatogonia (g) shows euchromatic
nucleus with coarse clumps of heterochromatin (ch). Note the Sertoli cell with
its triangular indented euchromatic nucleus (N) and prominent nucleolus (n).
TEMX8000.
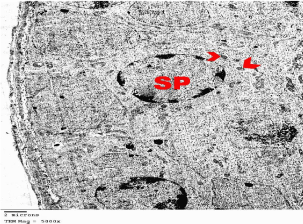
Figure 1: Electron microscopy of the testis of control group showing primary
Spermatocytes (SP) with large rounded nucleus. Its cytoplasm contains
mitochondria (arrow heads). TEMX5000.
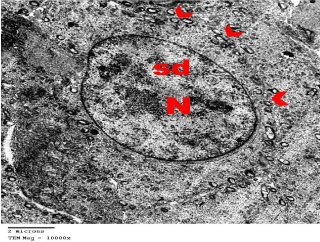
Figure 5: Electron microscopy of the testis of control group showing
spermatids (sd) with large spherical Nucleus (N). Cytoplasm appears granular
and shows vesicular mitochondria (arrow heads). TEMX10000.
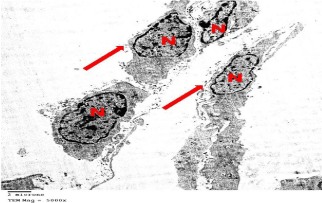
Figure 6: Electron microscopy of testis of control group showing an interstitial
Leydig cells having slightly elongated indented Nucleus (N) with peripheral
heterochromatin. Note the cytoplasmic processes (arrows). TEMX5000.
Ultrastructural examination of SCH group revealed destructed spermatogonia and Sertoli cells that showed condensed nuclei and were rested on irregular thick basement membrane (Figure 8). Wide separations in-between the germ cells was observed (Figures 8-10). Some spermatocytes had shrunken nuclei, electron dense bodies and vacuoles that were variable in size and electron density (Figure 9). Many spermatids appeared with no nuclei and multiple cytoplasmic vacuolation (Figure 10). Leydig cell population showed irregular shaped heterochromatic nuclei and multiple vacuoles. Leydig cells were surrounded by a large amount of collagen fibers (Figure 11). Mid pieces of sperms showed markedly affected disarranged axoneme, fibrous and mitochondrial sheaths (Figure 12).
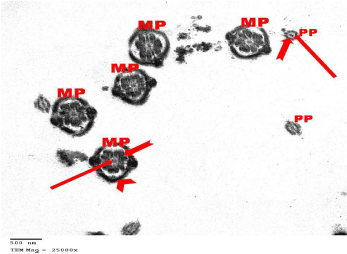
Figure 7: Electron microscopy of the testis of control group showing cross
sections in the Mid Pieces (MP) and Principal Pieces (PP) of the sperms. All
pieces have a central axoneme (arrows). In the mid pieces, the axoneme is
surrounded by a fibrous sheath (notched arrow) and mitochondrial sheath
(arrow head). The axoneme of the principle pieces is surrounded by the
fibrous sheath only (notched arrow). TEMX25000.
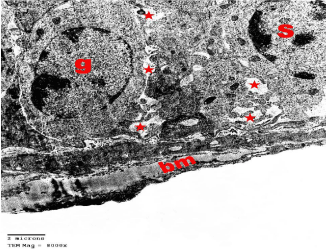
Figure 8: Electron microscopy of the testis of SCH rat showing nuclear
condensation of sprimatogonia (g) and Sertoli cell (S) that rest on an irregular
thick basement membrane (bm). Note many wide intercellular spaces (*).
TEMX8000.
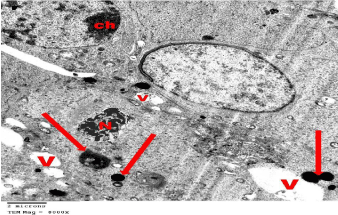
Figure 9: Electron microscopy of the testis of SCH rat showing spermatocytes
with numerous Vacuoles (V) that is variable in size. One spermatocyte shows
shrunken Nuclei (N) and electron dense bodies (arrows). Other one shows
heterochromatin clumps (ch). TEMX8000.
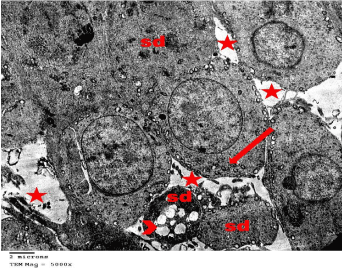
Figure 10: Electron microscopy of the testis of SCH rat showing Spermatids
(Sd) with no apparent nucleus and multiple cytoplasmic vacuolation (arrow
head). Abundant mitochondria (arrow) were localized close to the cell
membrane. Wide intercellular spaces (*) was obviously noted. TEMX5000.
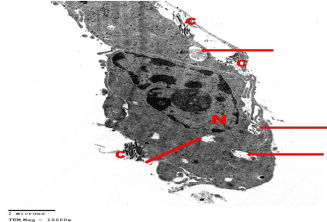
Figure 11: Electron microscopy of the testis of SCH rat showing Leydig cell
with an irregular shaped heterochromatic nucleus (N) and multiple vacuoles
(arrows). Note a large amount of Collagen fibers (C) that surrounded the cell.
TEMX10000.
Treated rats showed that they regained nearly their normal general architecture. Spermatogonia, primary spermatocytes, spermatids, sperms and sertoli cells showed their normal fine structure. However, intercellular separations were still present between some cells (Figure 13).
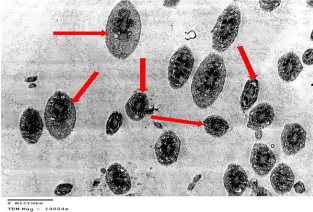
Figure 12: Electron microscopy of the testis of SCH rat showing distortion of
the axoneme, fibrous and mitochondrial sheaths in the middle piece of the
sperms (arrows). TEMX10000.
Haematoxylin and Eosin (H&E) stain for thyroid sections: In the control group, thyroid parenchyma was composed of different sized follicles. The follicular walls were lined by a single layer of flattened to cuboidal follicular cells, with flat to oval nuclei. The follicular lumens were filled with homogenous acidophilic colloids that had peripheral small vacuoles. An apparent few number of interfollicular cells and blood capillaries were observed in between the follicles (Figure 14A and B).
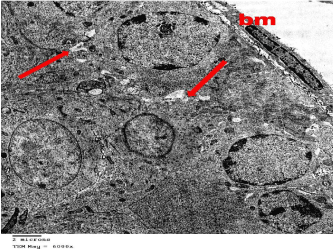
Figure 13: Electron microscopy of the testis of treated rat showing that
spermatogenic cells showed their normal fine structure. The basement
membrane (bm) is thin and regular. Intercellular separations (arrows) was
still present between some cells. TEMX6000.
In SCH group, sections from the thyroid glands showed that most of the follicles were apparently distended with vacuolated colloid whereas others showed desquamated follicular cells in their lumen. Congested blood capillaries were also observed. Some follicles appeared with no colloid in their lumens and were lined by cuboidal vacuolated cells. Other follicles were lined by multiple layers of vacuolated follicular cells. Signs of mitosis were observed in the follicular epithelial lining (Figure 14B and C).
Sections of the treated rats showed almost normal organized glandular tissue. Few follicles were distended with vacuolated colloids (Figure 14E and F).
Immunohistochemical stain for thyroid sections
The SCH group dramatically down-regulated the expression of Thyroglobulin as compared with control group. Ginseng coadministration significantly increased the expression of Thyroglobulin (Figure 15).
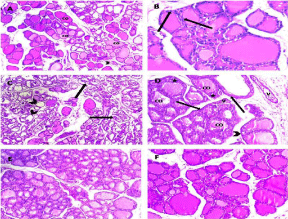
Figure 14: Representative H&E staining of rat thyroid gland of different
experimental groups. The section of the thyroid gland in the control group
showed thyroid follicles of different sizes; their cavities contained vacuolated
acidophilic Colloid (CO). Blood capillaries (arrow head) were seen in the
connective tissue between follicles (A). Follicular wall was lined mostly with
flat follicular cells with flat to oval nuclei (arrows, B). In the treated group,
there was loss of normal glandular architecture. Sections showed thyroid
follicles devoid of the colloid in their lumen (arrow heads), others showed
desquamated follicular cells in their lumen (arrows, C). Most follicles were
distended with vacuolated Colloids (CO). Follicles were lined by multiple
layers of follicular cells (*) and vacuolation in their cytoplasm (arrow head).
Dilated congested blood Vessels (V) were also Noted (D). Sections of the
treated rats showed significant improvement of histological architecture
with almost normal organized glandular tissue however, a few follicles were
distended with vacuolated colloids (E, F). Scale bar = 200 μm (A, C, E) and
100 μm (B, D, F).
Electron microscopic examination of thyroid gland: The examination of thyroid glands of group I (control group) revealed multiple follicles, each lined by a single layer of cuboidal follicular cells with large oval euchromatic nuclei. Their cytoplasm revealed a well-developed rough Endoplasmic Reticulum (rER), mitochondria and lysosomes. The apical border of the follicular cells exhibited a moderate number of short microvilli projected into the lumen filled with moderately dense colloid. Typical junctional complexes were seen between the adjacent follicular cells (Figure 16).
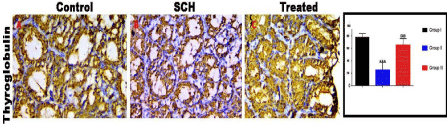
Figure 15: Expression of thyroglobulin receptors of experimental groups. Ginseng significantly up-regulated the SCH induced decrease in expression of
Thyroglobulin. ***p < 0.001, compared to the control group; and 000p < 0.05 compared to the SCH group. Scale bar = 100μm.
Electron microscopic examination of the ultrathin sections of the thyroid gland of SCH group revealed evident histological alterations of the follicles. Most of the follicular cells were flattened. The nuclei of follicular and interfollicular cells became heterochromatic. They were either flattened or relatively rounded with marked irregularity in their nuclear envelope (Figure 17). Collagen fibers were seen in the interstitial spaces between the follicles (Figures 17-20). The most prominent ultrastructural feature of the follicular cells was the distorted apoptotic nuclei along with cytoplasmic rarefaction and vacuolation and apparent increase in the lysosomal content of the follicular cells. Many follicles showed a few sparse or focal loss of apical microvilli at the luminal border (Figure 18). Many cells appeared with a dark irregular nucleus, vacuolated cytoplasm and widening of the junctional complexes between the follicles; associated frequent mast cells and collagen fibers were also detected (Figure 19).

Figure 16: Electron microscopy of a thyroid follicle of control group showing a
single layer of follicular cells surrounding a lumen filled with moderately dense
Colloid (CO). The cells show large euchromatic Nuclei (N) and numerous
short microvilli (arrows) projecting into the lumen. The cytoplasm reveals a
well-developed rough Endoplasmic Reticulum (rER), mitochondria (m), a
few Vacuoles (V) and dense Lysosomal granules (L). Note the junctional
complexes between adjacent follicular cells (J). TEMX12000.

Figure 17: Electron microscopy of a thyroid follicle of SCH group showing
follicular and interfollicular cells with flattened and irregular shaped
heterochromatic Nuclei (N). Note Collagen fibers (C) in the interstitial spaces
between the follicles. TEMX5000.
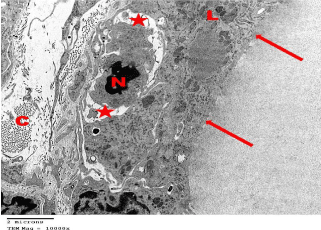
Figure 18: Electron microscopy of SCH group showing thyroid follicular
cells with focal loss of apical microvilli (arrows), apoptotic Nucleus (N),
numerous dense Lysosomes (L), rarified and vacuolated cytoplasm (star).
Note the interfollicular connective tissue with abundant Collagen fibers (C).
TEMX10000.
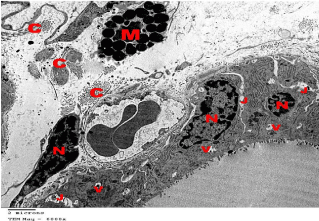
Figure 19: Electron microscopy of SCH group showing irregular dark Nuclei
(N) of follicular and interfollicular cells with Vacuolated cytoplasm (V) and
gaping of the Junctional complexes between adjacent follicular cells (J).
Prominent Mast cells (M) and excess Collagen fibers (C) were present in the
interfollicular spaces. TEMX6000.
Electron microscopic examination of treated group showed restoration of normal histological structure of thyroid follicular cells however, few collagen fibers were present in the interfollicular spaces (Figure 20).
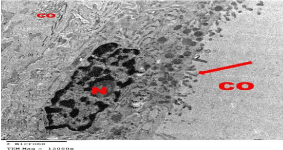
Figure 20: Electron microscopy of treated group showing restoration of
normal histological structure of follicular cells that surrounded a lumen filled
with moderately dense Colloid (CO). The cells show large euchromatic Nuclei
(N) and numerous short microvilli (arrows) projecting into the lumen however,
few Collagen fibers (C) were still present in the interfollicular spaces.
TEMX12000.
Discussion
The testicular function is the mirror of thyroid gland state as disturbance of the normal euthyroid state affects the structure and function of the testis. The subclinical hypothyroidism is a risky condition that may progress toward overt hypothyroidism [15], also SCH was found to be independently associated with an increased risk of all causes of mortality [16]. Both subclinical and overt hypothyroidism adversely affects the testicular function [1]. Our study is the first to describe a novel effect of propylthiouracil induced subclinical hypothyroidism on the function and structure of adult male albino rat testis and to evaluate the possible protective role of ginseng. The results of epididymal spermatozoa study of SCH group showed a statistically significant decrease in the mean sperm count, viability and motility when compared with the control group, also the ultrastructure examination of testicular sections of SCH group revealed disarranged axoneme of mid pieces, fibrous and mitochondrial sheaths of spermatozoa. This is in accordance with the result of other authors who stated that propylthiouracil induced overt hypothyroidism resulting in impairment of reproductive efficiency of adult male rats [17].
The testicular section of SCH group showed irregular outlines and maturation arrest in several seminiferous tubules. Other tubules showed sloughed germinal epithelial cells in the lumina and multiple giant cell formation. In other fields many of the spermatocytes appeared with pyknotic nuclei surrounded by a halo indicating apoptosis and the interstitial connective tissue showed acidophilic hyaline material with dilated congested blood vessels.
The ultrathin testicular sections of SCH group revealed this damage that also included vacuolated leydig cell populations which were surrounded by a large amount of collagen fibers. These collagen fibers explained the hyalinized appearance of the interstitial tissue that was revealed by light microscope. This observed testicular damage was explained by lipid peroxidation and excessive production of Reactive Oxygen Species (ROS) indicating the oxidative stress [18]. As the hypothyroidism markedly increased the proteins carbonylation which disturbed the redox status and compromised the antioxidant defence system [19]. Testosterone signalling through Androgen Receptor (AR) is vital for complete spermatogenesis and this is mediated by the somatic cell types of the testis. These cells included specifically the Sertoli and peritubularmyloid cells that have intimate connections with the maturing germ cells which showed also the expression of AR [20,21]. So, the decreased expression of androgen receptor indicates the impairment of spermatogenesis as well as testosterone production [17].
Regarding the thyroid sections of SCH group, some follicles were distended with vacuolated colloid whereas others showed desquamated follicular cells in their lumen. The electron microscopic examination of the ultrathin thyroid sections of SCH group also illustrated these marked follicular alterations which included distorted apoptotic nuclei along with cytoplasmic rarefaction and apparent increase in the lysosomal content of the follicular cells. This is in agreement with Gosseli et al., who denoted these changes of hypothyroid follicles to the circulating antibodies [22]. Moreover, AbdAllah, 2013 stated that hypothyroidism resulted in excessive production of reactive oxygen species; oxidative stress and subsequently lipid peroxidation and cell death followed by the desquamation of the degenerated follicular cells and added that vacuolated colloid results from a defect in pinocytosis of thyroglobulin follicular cell [23].
The multiple layers of the follicular epithelium, glandular congestion and empty follicles were recorded by light microscope, together with increased mast cell in the follicular cells that was revealed by electron microscope. All the previous changes were explained by increased TSH release from the pituitary gland which leads to hypertrophy followed by hyperplasia of thyroid epithelium [24].
Thyroglobulin (TGB) is produced by the thyroid gland, acting as a precursor of thyroid hormones (T3 and T4), and serving as a specific marker for thyroid follicular cell. TGB expression is diffuse in 100% of normal thyroid follicular epithelial cells [25]. The decreased thyroglobulin expression indicates the impaired function of the thyroid gland [26]. This is in agreement with our result, as thyroid sections of SCH group showed significant down regulation of the thyroglobulin expression.
However, ginseng treatment protected the testis and thyroid from most of the above changes except some testicular cells showed pyknotic nuclei and some follicles showed vacuolated colloid, this partial protection was explained by the antioxidant mechanisms to combat ROS production partially or totally through prevention of peroxidation of substrate by the endogenous antioxidant enzymes such as SOD, GSH-Px and CAT are mainly responsible for the elimination of excessive ROS [27]. This was in agreement with other authors who stated that garcinia kola and vitamin E showed slight improvement in the damaged testes with Lead II oxide when compared with Group C that received Lead II oxide only [28]. The testicular protection property of ginseng was explained by the presence of ginsenosides which are the main active compounds in ginseng [29]. Ginsenoides were considered as powerful antioxidants reducing the oxidative stress and delaying the aging [30]. Beside the antioxidant properties of the ginseng, it is considered as a thyromimetic agent as reported by Filho et al. [30]. This thyromimetic effect is implicated in the up regulation of thyroglobulin expression in the thyroid sections of ginseng treated group. So we concluded that the subclincal hypothyroidism led to reproductive deficiency that was detected biochemically, histologically and immunohistochemically. This deficiency could be reversed by ginseng usage which was considered as antioxidant and thyromimetic agent that protected the thyroid and the testis from the effects of SCH.
References
- Wagner MS, Wajner SM, Mala AL. Is there a role for thyroid hormone on spermatogenesis? Microsc Res Tech. 2009; 72: 796-808.
- Ge JF, Xu YY, Li N, Zhang Y, Qiu GL, Chu CH, et al. Resveratrol improved the spatial learning and memory in subclinical hypothyroidism rat induced by hemi-thyroid electro cauterization. Endocrine Journal. 2015; 62: 927-938.
- Chiao CY, Lin H, Wang WS, Wang SP. Direct effects of propylthiouracil on testosterone secretion in rat testicular interstitial cells. Br J Pharmacol. 2000; 130: 1477-1482.
- Ge JF, Peng L, Hu CM, Wu TN. Impaired learning and memory performance in subclinical hypothyroidism rat model induced by hemi-thyroid electro cauterization. Journal of Neuroendocrine. 2004.
- Nakamura H, Noh JY, Itoh K, Fukata S, Miyauchi A, Hamada N. "Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by graves’ disease". J Clinic Endocrinol Metab. 2004; 92: 2157-2162.
- Sener G, Kabasakal I, Atasoy BM, Erzik C, Velioglu OA, Cetinel S, et al. Propylthiouracil induced hypothyroidism protects ionizing radiation-induced multiple organ damaging rats. J Endocrinol. 2006; 189: 257-269.
- Park SJ, Lim KH, Noh JH, Jeong E, Kim JH, Han BC, et al. Moon subacute oral toxicity study of korean red ginseng extract in sprague-dawley rats. Toxicol Res. 2013; 29: 285-292.
- Jang M, Woo J, Jun M, Chun GD. Effects of red ginseng extract on the epididymal sperm motility of mice exposed to ethanol international. Journal of Toxicology. 30: 435-442.
- Whitehead TP, Thorpe GH, Carter TJ, Kricka LJ. Enhanced luminescence procedure for sensitive determination of peroxidase labeled conjugate in immunoassay. Nature. 1983; 305: 158-159.
- Burtis CA, Ashwood ER, Bruns DA. Principles of immunochemical techniques. Saunders Company. 1994; 1715-1730.
- Stirling JW, Woods A, Bancroft JD, Gamble M. Transmission electron microscopy: diagnostic applications. Theory and practice of histological techniques. Churchill Livingstone. 2002; 701-728.
- Pearl CA, Mason H, Roser JF. Immunolocalization of estrogen receptor alpha, estrogen receptor beta and androgen receptor in the pre-, peri- and post-pubertal stallion testis. Anim Reprod Sci. 2011; 125: 103-111.
- Kirkpatrick LA, Feeney BC. A simple guide to IBM SPSS statistics for version 20.0. Cengage Learning. 2013.
- Bona G, Prodam F, Monzani A. "Subclinical hypothyroidism in children: natural history and when to treat". Journal of Clinical Research in Pediatric Endocrinology (Review). 2013; 1: 23-28.
- McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study. Thyroid. 2011; 21: 837-843.
- Alkalby JMA, Alzerjawi SJS. Effect of propylthiouracil–induced hypothyroidism on reproductive efficiency of adult male rats. Bas j vet Res. 2013; 12: 2.
- Ilbey Y, Zbek ES, Ims EKA, Ekmen M, Somay A. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertil Steril. 2009; 92: 1124-1132.
- Sahoo DK, Roy A, Chainy GBN. “PTU-induced neonatal hypothyroidism modulates antioxidative status and population of rat testicular germ cells”. National Academy Science Letters. 2006; 29: 133-135.
- Vornberger W, Prins G, Musto NA, Suarez-Quian CA. Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. Endocrinology. 1994; 134: 2307-2316.
- Hara LO, Smith LB. Androgen receptor roles in spermatogenesis and infertility. Best Practice & Research Clinical Endocrinology & Metabolism. 2015; 29: 595-605.
- Gosselinc SJ, Capen C, Martin SL. Histologic and ultrastructural evaluation of thyroid lesions associated with hypothyroidism in dogs. Vet Pathol. 1981; 18: 299-309.
- AbdAllah MA. Effect of bilateral orchidectomy on thyroid gland structure of adult albino rats and the role of nandrolone decanoate administration. Journal of American Science. 2013; 9.
- Shelke VM, Pathak VP, Bedre DK, Patil JM, Mote CS. Study of histopathological changes in thyroid gland in buffaloes. Veterinary World. 2009; 2: 387-389.
- Bejarano PA, Nikiforov YE, Swenson ES, Biddinger PW. Thyroid transcription factor-1, thyroglobulin, cytokeratin 7, and cytokeratin 20 in thyroid neoplasms. Appl Immuno histo chem Mol Morphol. 2000; 8: 189-194.
- Lima MA, Gontijo VA, Schmitt FCL. Thyroid peroxidase and thyroglobulin in normal human thyroid glands expression. Endocrine Pathology. 1998; 9: 4.
- Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000; 153: 83-104.
- Dare BJ, Chukwu RO, Oyewopo AO, Makanjuola VO, Olayinka PO, Akinrinade ID, et al. Histological integrity of the testis of adult wistar rats (rattusnovergicus) treated with garcinia kola. Reprod Sys Sexual Disorders. 2012; 1: 4.
- Hwang SY, Sohn SH, Wee JJ, Yang JB, Kyung JS, Kwak YS, et al. Panax ginseng Improves Senile testicular function in Rats. J Ginseng Res. 2010; 34: 327-335.
- Saw CLL, Yang AY, Cheng DC, Boyanapalli SS, Su ZY, Khor TO, et al. Pharmacodynamics of ginsenosides: antioxidant activities, activation of nrf2 and potential synergistic effects of combinations. Chem Res Toxicol. 2012; 25: 1574-1580.
- Filho GP, Graf H, Ward LS. Comparative analysis of the new guidelines and consensuses for the management of hypothyroidism, thyroid nodules, and differentiated thyroid cancer. Arq Bras Endocrinol Metab. 2013; 57: 4.