
Research Article
Austin J Anat. 2017; 4(2): 1068.
Histological and Immunohistochemical Studies on the Cornea and Retina of Sofosbuvir Treated Rats
Issa NM and El-Sherif NM*
Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Egypt
*Corresponding author: El-Sherif NM, Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Egypt
Received: February 09, 2017; Accepted: March 06, 2017; Published: March 16, 2017
Abstract
Sofosbuvir (Sovaldi) is used in treatment of hepatitis C infection. The aim of this study is to demonstrate the effect of Sofosbuvir on the cornea and retina of adult male albino rat. Twenty adult male albino rats were used in this experiment. Animals were divided in two groups: Group I (Control) included ten rats were kept without any treatment throughout the experimental period and Group II (Sovaldi treated) included ten rats that received Sofosbuvir in the form of tablets with the trade name ‘Gratisovir’. Each tablet contained 400 mg of Sofosbuvir; these tablets were dissolved in distilled water and given for rats orally by gastric tube in a dose of 4mg/kg per day for 5 weeks. Sofosbuvir results in histological and immunohistochemical changes in cornea and retina of Sovaldi treated rats.
Keywords: Sofosbuvir; Cornea; Retina; E-cadherin; Fibronectin
Introduction
Sofosbuvir is a nucleotide analogue polymerase inhibitor with the same in vitro activity against all hepatitis c virus genotypes. Before the discovery of Sofosbuvir, different nucleoside analogs had been tested as antihepatitis C treatments, but these showed relatively low potency. This low potency comes in part because the enzymatic addition of the first of the three phosphate groups of the triphosphate is slow. The structure of Sofosbuvir, based on the protide approach, avoids this slow step by building the first phosphate group into the design of the drug during synthesis. Additional groups are attached to the phosphorus to temporarily mask the two negative charges of the phosphate group, thereby allowing entry of the drug into the infected cell [1]. Sofosbuvir and other nucleotide inhibitors of the HCV RNA polymerase show great difficulty for resistance development. This is an important advantage relative to other drugs of HCV that target other viral enzymes such as the protease, for which rapid development of resistance occurred, that lead to therapeutic failure [2]. It was reported that the Sovaldi treatment displays common side effects as chest pain, migraine, memory impairment, dyspnea, gastrointestinal reflux, alopecia, depression, muscle spasm and blurred vision [3]. Moreover vision loss seems to be a growing side effect of Sovaldi [4].
Methodology
Animals
Twenty adult male albino rats were used in this experiment, each of them is weighting 170-200 grams were used in this experiment. Food and water were provided ad libitum for one week before use in the anatomy department, faculty of medicine, Menoufia University. All aspects of animal care and treatment were carried out according to the local guidelines of the ethical committee for animal research.
Experimental design
The animals were divided in two groups: Group I (Control): included ten rats were kept without any treatment throughout the experimental period.
Group II (Sovaldi treated): included ten rats that received Sofosbuvir (Sofosbuvir, a product of Pharco Pharmaceuticals, Alexandria, Egypt, was available in the form of tablets with the trade name ‘Gratisovir’. Each tablet contained 400 mg of Sofosbuvir; these tablets were dissolved in distilled water and given for rats orally by gastric tube in a dose of 4 mg/kg per day for 5 weeks.
Histological study
Light microscope: The specimens of the two organs from the two groups were fixed in 10% neutral-buffered formalin, dehydrated in a graded ethanol series, cleared in xylene, and embedded in paraffin wax. Tissue sections of 5–7 μm thickness were cut using a rotating microtome, deparaffinized with xylene, and stained with H&E for LM examination of the corneas and retinas.
Immunohistochemical stains: Five micrometer sections were mounted, dried, and dewaxed in xylene. Rehydrated sections were then incubated with 3% hydrogen peroxide in humidified boxes to block the endogenous activity of peroxidase. Microwave-assisted antigen retrieval was performed for 20 min. Sections were then incubated overnight at 4C with:
1. Primary mouse monoclonal antirat E-cadherin antibody, (NeoMarkers/Lab Vision, Fremont, California, USA).
2. Primary rabbit antirat fibronectin antibody, (NeoMarkers/ Lab Vision, Fremont, California, USA) (Mouse monoclonal antibody).
After washing with phosphate-buffered solution, sections were incubated with biotinylated antirabbit secondary antibody for 30 min and then with streptavidin peroxidase conjugate for 30 min. Sections were then washed with phosphate buffer solution, and incubated with diaminobenzidine chromogen to detect immunoreactivity. Counterstaining was performed by Mayer’s hematoxylin. Positive reaction was visualized as brown coloration.
Morphometric and statistical analysis
A detailed morphometric analysis of the area percent of fibronectin and e cadherin expression was assessed in the ocular tissues of the different sections from each slide of different animals at a magnification of x400. This was performed using a Leica Qwin 500 MCO image analysis system (Wetzlar, Germany). Values of the measured parameters were presented as mean±standard deviation. The statistical analysis was processed according to the conventional procedures using the Statistical Program of Social Sciences (SPSS) software for windows (version 10.0) (IBM, USA).
Results
Cornea results
H&E light microscopy: Corneal examination of the control group showed normal histological structure formed of many layers: the outer stratified squamous nonkeratinized corneal epithelium, Bowman’s membrane, the stroma (substantia propria) and Descemet’s membrane (Figure 1). Corneal examination of SOV treated group showed focal areas of desquamation and separation of the epithelium. Some areas showed loss of corneal epithelium with apparent decrease in thickness of the epithelium. Corneal stromal collagen bundles were separated by multiple wide spaces, indicating corneal edema and showed cellular infiltration (Figures 2 and 3).
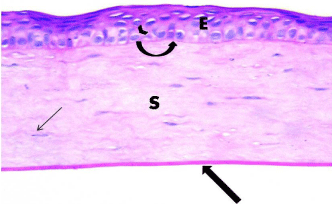
Figure 1: Photomicrograph of a section of rat cornea from the control
group, showing the stratified squamous nonkeratinized corneal Epithelium
(E) formed of a few cell layers. The basal epithelial cells are columnar with
oval nuclei (arrow head) resting on Bowman’s membrane (curved arrow).
The Stroma (S) is formed of parallel arranged collagen bundles with spindleshaped
keratocytes in between (thin arrow). Thick arrow points to Descemet’s
membrane. H&E × 400.
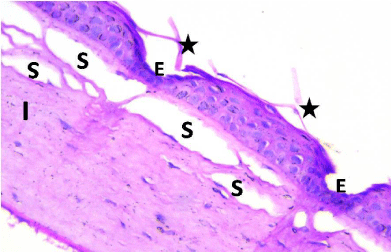
Figure 2: Photomicrograph of a section of rat cornea from the SOV group,
revealing desquamation and separation of the epithelium (Star). Some areas
showed loss of corneal epithelium in some areas and apparent decrease in
thickness of the Epithelium (E); corneal stroma fibers were widely Separated
(S) and showed cellular Infiltration (I). H&E × 400.
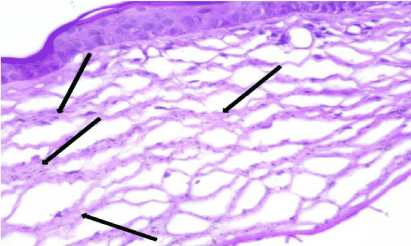
Figure 3: Photomicrograph of a section of rat cornea from the SOV group,
showing thick disrupted widely separated collagen fibers of the stroma
(Arrows). H&E × 400.
Immunohistochemical assaying showed that SOV treatment significantly down-regulated the expression of E-cadherin as compared to the control group (Area % 78.23±1.83 Vs 23.18±6.45) (Figure 4).
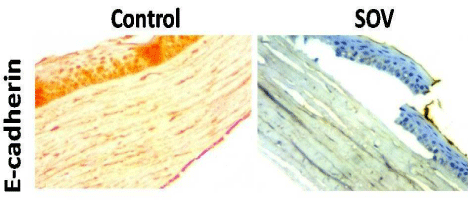
Figure 4: Expression of corneal E-cadherin in control and SOV-treated
groups. SOV significantly down-regulated the expression of E-cadherin as
compared to the control group. H&E × 400.
Retina results
Examination of haematoxylin and eosin stained sections of the retina in the control group (group I) showed normal histological structure demonstrating the photoreceptor layer, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer and optic nerve layer. The outer nuclear layer exhibited darkly stained nuclei whereas the inner nuclear layer and ganglion cell layer showed lightly stained vesicular nuclei (Figures 5-8). Sections of SOV treated group showed denuded pigmented epithelium in the photoreceptor layer and fissures in the outer nuclear layer. Extravasated Red Blood Corpuscles (RBCs) were noted in the inner nuclear layer and ganglion cell layer. Vacuoles and wide clear areas were present around ganglion cells that showed irregular dark nuclei (Figures 9-12). SOV treatment significantly up-regulated the expression of fibronectin immunostaining compared to the control group (Area % 14.68±1.45 Vs 72.76±4.16) (Figure 13).
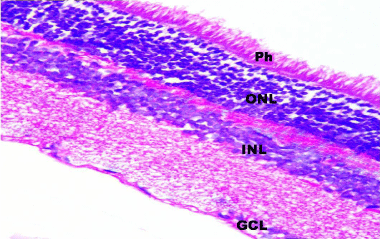
Figure 5: A photomicrograph of a section in rat retina of control group
showing the normal histological layers of the retina; Photoreceptor layer (Ph),
Outer Nuclear Layer (ONL), Inner Nuclear Layer (INL) and Ganglion Cell
Layer (GCL). H&E, × 400.
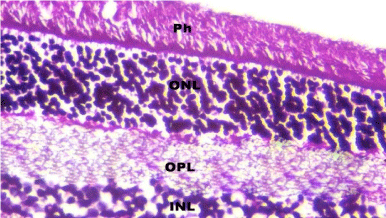
Figure 6: A photomicrograph of a section in rat retina of control group
showing the Photoreceptor layer (Ph), outer nuclear layer with darkly stained
nuclei (ONL), Outer Plexiform Layer (OPL) and inner nuclear layer with lightly
stained nuclei (INL). H&E, × 560.
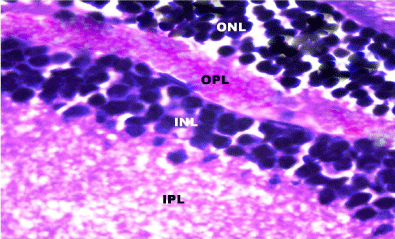
Figure 7: A photomicrograph of a section in rat retina of control group
showing the Outer Nuclear Layer (ONL), Outer Plexifrom Layer (OPL), Inner
Nuclear Layer (INL) and Inner Plexiform Layer (IPL). H&E, × 1000.
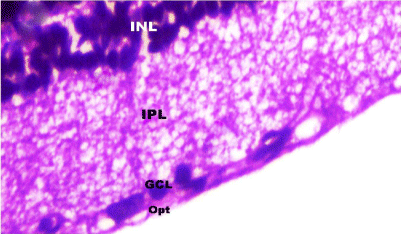
Figure 8: A photomicrograph of a section in rat retina of control group
showing the Inner Nuclear Layer (INL), Inner Plexiform Layer (IPL), Ganglion
Cell Layer with lightly stained nuclei (GCL) and Optic nerve layer (Opt). H&E,
× 1000.
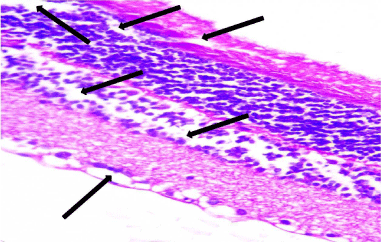
Figure 9: A photomicrograph of a section of the retina from an adult albino rat
from the SOV group showing disorganized disrupted architecture of all retinal
layers with widely separated cells (arrows). H&E, × 400.
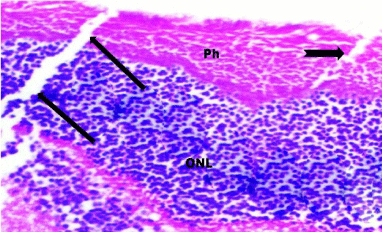
Figure 10: A photomicrograph of a section of the retina from the SOV group
showing focal area of denuded pigmented epithelium (notched arrow), and
a fissure (arrows) in the Photoreceptor layer (Ph) that extended to the Outer
Nuclear Layer (ONL). H&E, × 560.
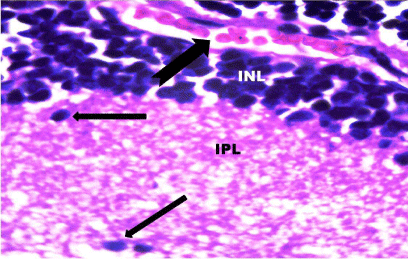
Figure 11: A photomicrograph of a section of the retina from the SOV group
showing extravasation of blood (notched arrow) in the Inner Nuclear Layer
(INL). The Inner Plexiform Layer (IPL) demonstrated irregular nuclei (arrows).
H&E, × 1000.
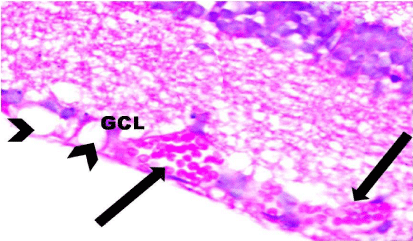
Figure 12: A photomicrograph of a section of the retina from the SOV group
showing dilated congested blood vessels (arrows) and vacuolation (arrow
heads) in the Ganglion Cell Layer (GCL). H&E, × 1000.
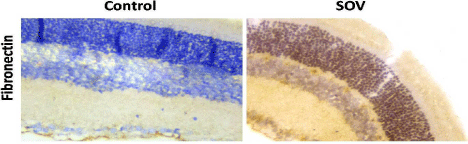
Figure 13: Expression of retinal fibronectin in control and SOV-treated
groups. SOV significantly up-regulated the expression of fibronectin
immunostaining compared to the control group. H&E, × 560.
Discussion
We aimed in this study to clarify the corneal and retinal causes of the blurred vision presents on the Sofosbuvir administration, the Heamatoxyline and Eosine stained sections revealed multiple changes including desquamation, loss of corneal epithelium; which was attributed to breakdown of tight junctional integrity in the epithelial cells allowing direct passage of hydrophilic molecule through paracellular space to the cornea [5], which explained by marked reduction of a tight junction-related protein (zonula occludin-1) was markedly reduced in epithelial cells followed by disruption of epithelial barrier functions [6]. It was reported that the corneal epithelium is a good sensor which may reflect functional alterations of the cell membrane integrity and was considered as a manifestation of drug toxicity [7]. Our result also revealed wide separation of stromal collagen fibers with cellular infiltration, the cations reaction with the carboxyl groups of stromal collagen and glycoaminoglycans causing disarrangement to the collagen bundles as explained by Maurer et al., 2001 [8]. The keratocytes implicated in maintaining the highly organized collagen fibrils and the intercellular matrix, more keratocytes means better function [9]. E-cadherin is an essential adhesion molecule that maintains the corneal epithelial structural and functional integrity; the reduction of E-cadherin expression mostly indicated the altered integrity of the epithelial cell layers with impaired packing and organization of the cells [10]. This is in agreement with our result including reduction of E-cadherin expression together with sloughing of the epithelium in Sofosbuvir treated group. Disturbance of epithelial integrity could lead to impairment of its barrier function, this exposes the corneal stroma to disruption of its collagen bundles causing severe defect of vision due to loss of its transparency and so light refraction [11].
Regarding the retinal changes that occurred to Sofosbuvir treated group included disrupted architecture of all retinal layers with widely separated cells, denuded pigmented epithelium, fissured photoreceptor layer that extended to the outer nuclear layer with dilated congested blood vessels with extravasation of blood and vacuolation in the ganglion cell layer. These changes were explained by breakdown of the blood-retinal barrier which is an early functional disorder that can cause leakage from the vessels; retinal edema and so disturbed architecture which in turn results in visual disturbance [12]. Some authors suggest that the onset of blood retinal barrier disturbance may coincide with the degeneration of the retina and activation of the microglial cells [13].This is in agreement with our result which included the up regulation of fibronectin expression in the retina of Sofosbuvir treated rats; this might be explained by the special affinity of the fibronectin to collagen, heparin, fibrin, fibrinogen, which already plenty extravasated due to disturbance of blood retinal barrier. So Sofosbuvir may be implicated in the occurrence of the retinopathy, this explains the blurring of vision that accompany Sofosbuvir administration.
References
- Murakami E, Tolstykh T, Bao H, Niu C, Steuer HM, Bao D, et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J Biol Chem. 2010; 285: 34337-34347.
- Eric L, Alessandra M, David W, ZeidKayali L. Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection. N engl j med. 2013; 368-320.
- FDA. U.S. Food and Drug Administration. FDA Drug Safety Communication. 2015.
- Andrasko G, Ryen K. Corneal staining and comfort observed with traditional and silicone hydrogel lenses and multipurpose solution combinations. Optometry. 2008; 79: 444-454.
- Amany M. Histological and Immunohistochemical Study on the Effect of Polyhexanide-Preserved Contact Lens Solution on Albino Rat Cornea and the Possible Protective Role of Carboxymethyl cellulose. Egypt J Histol. 2010; 33: 288-300.
- Geerling G, Daniels JT, Dart JK, Cree IA, Khaw PT. Toxicity of natural tear substitutes in a fully defined culture model of human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001; 42: 948-956.
- Maurer JK, Molai A, Parker RD, Carr GJ, Petroll WM, Cavanagh H, et al. Pathology of ocular irritation with acetone, cyclohexanol, parafluoroaniline and formaldehyde in the rabbit low-volume eye test. Toxicol Pathol. 2001; 29:187-199.
- Bantseev V, McCanna DJ, Driot JY, Sivak JG. The effects of toxicological agents on the optics and mitochondria of the lens and the mitochondria of the corneal epithelium. Semin Cell Dev Biol. 2008; 19:150-159.
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res. 2002; 74: 753-760.
- Dhruva J, Giuseppe F, Shelley S, Trevor W, Judith A. Deletion of AP-2a Leads to Disruption in Corneal Epithelial Cell Integrity and Defects in the Corneal Stroma. Invest Ophthalmol Vis Sci. 2005; 46: 3623-3630.
- Murata T, Ishibashi T, Inomata H. Immunohistochemical detection of extravasated fibrinogen (fibrin) in human diabetic retina. Graefes Arch Clin Exp Ophthalmol. 1992; 230: 428-431.
- Richard F, Sarah K, Dorit B. Maria K. Preclinical Retinal Neurodegeneration in a Model of Multiple Sclerosis. J Neurosci. 2012; 32: 5585-5597.
- Naglaa I. Immunohistochemical localization of fibronectin in the ocular tissues of adult male albino rat. Egypt J Histol. 2011; 34: 191-198.