
Research Article
Austin J Anat. 2017; 4(3): 1072.
Von Economo Neurons in Indian Green Ring Neck Parrot (Psittacula Krameri): Possible Role in Vocal Learning
Shubha S* and Sudhi S
¹Department of Zoology & Applied Aquaculture, Kashi Naresh Government Post Graduate College, India
²Department of Zoology & Applied Aquaculture, Barkatullaha University, India
*Corresponding author: Shubha S, Department of Zoology & Applied Aquaculture, Kashi Naresh Government Post Graduate College, Bhadohi, India
Received: April 14, 2017; Accepted: June 07, 2017; Published: June 14, 2017
Abstract
Only a few vertebrates communicate acoustically, notably the three birds’ group - songbirds, hummingbirds, and parrots; and some mammals – cetaceans, bats, elephants, seals, and hominids are able to produce elaborate patterns of vocalization. Vocal learning and Imitation is a rare trait and one of the highest forms of social learning. Here detailed anatomical investigation of the brain of Indian parrot - an exclusive imitator, reveal that brain wiring of parrot is unique with specialized cells and lacking multilayered cortical structure. We encounter an unusual neuronal type, known as Von Economo Neurons (VENs) in parrot – those have been noticed to date only in a few groups of mammals like humans, elephants, cetaceans, pinnipeds, and a few primates. Almost all are vocal learners. Functionally these neurons are associated with intelligence, cognition, emotion, facial expression as well as autonomic visceral, olfactory and gustatory functions. The presence of VENs in vocal counterparts of parrot pallium especially in the nidopallium caudolaterale (callopallial anterior region), lemnopallial region - the dorsolateral corticoid area (CDL), and in Arcopallium, indicate that parrot brain is restricted with compact nuclei and diversity of neural subtypes. Except VENs, Fork neurons and enveloping neurons have also been noticed in these regions. Strikingly similar Neuroarchitecture of vocal control areas of parrot brain to upper layer of mammalian neocortex provide clues of involvement of these neurons in the processing of vocal communication and imitation. These findings are important to understand the common blueprint of brain wiring of high-level cognition during evolution and related neurodegenerative disorders.
Keywords: Fork neuron; Lateral pallium; Indian parrot; Vocal learning; Neuroarchitecture; Von economo neuron
Introduction
Human language is a unique and complex phenomenon, of the combination of imitation, memory, thought, emotions, logic, syntax, reasoning etc, and requires an enormous amount of brain resource. These resources are necessary to assemble information, syntactic constructions of thousands of words and their interconnections. The neuronal components of all these brain resources are still not fully known. Some of them we can definitely find with animal communication. Vocal production learning and imitation (the substrate for human language) one of the highest forms of social learning, is a rare and critical trait appears to have evolved independently in restricted groups of birds and mammals. Certain distantly related groups of large brain mammals –humans, cetaceans [1], elephants [2,3] harbor seals [4], dolphins [4,5] and pinnipeds (walrus) [6] can learn to produce elaborate patterns of vocalizations and can grasp most the meaning of sound. This rare ability is also reported dramatically in some small brain mammals, such as pygmy hippopotamus, manatee [7], ring- tailed lemur [8] pig [9,10], Deer [11], macaque monkey [12], rock hyrax [13], goat [14], and cattle [15]. In mice (Musmusculus) varying level of complex calls have been noticed [16]. Vocal communication in bats has also been studied extensively [17].
A few bird groups- songbirds, hummingbirds, and parrots also have the ability to imitate sounds. The Parrot family other than humans, cetaceans, and elephants etc. has shown the capability of extensive and rich vocal imitation [18-20] that can imitate human speech without training or an obvious reward [21]. Moreover, parrots are also found having emotions, cognition and social intelligence [22].
Surprisingly large, spindle -shaped bipolar projection neurons the Von Economo Neurons (VENs) are discovered in the layer V of the Anterior Cingulate Cortex (ACC) and Fronto Insular cortex (FI) of same groups of animals- humans, great apes [23-26], cetaceans [27- 28] elephants [29], in anterior insula of macaque monkey [30-11], pygmy hippopotamus, the Atlantic walrus, Florida manatee, zebra, horse pig, [31-32], rock hyrax, and white- tailed deer [33]. Almost all these species have shown capabilities of vocal production learning. VENs are considered as unique neurons due to their confined presence, restricted location, and specific morphology. Functionally VENs in great apes has been associated with social cognition and selfawareness, in cetaceans and elephants these cells have been correlated with brain size and the ‘social brain’. The speculation that VENs are recently emerged specialization to facilitate rapid information processing in large size brain is also not seemingly true as these cells have also been reported in comparatively smaller brain, like- lemurs [34] pygmy hippopotamus [31-32], the Florida manatee, [35] as well as macaque monkey [30,11].
The emergence and localization of high density of these specialized neurons in Anterior Insula (AI), and ACC of humans suggests their role in the development of intelligent behavior [24], integration of emotions, facial expression, [36], social conduct [37], as well as regulation of autonomic visceral, olfactory, and gustatory functions [38] with highly uncertain situations [39], salience evaluation [40] and self- regulation [36,41]. The vulnerability of VENs in pathological Dementia (Social behavior deficit), autism (language deficit), [36] and neuropsychiatric and neurodegenerative disorder (Alzheimer’s) suggest their role in social behavior [37]. Certain forms of human dementia that involve loss of self-awareness, perspective talking, and empathy are also correlated with loss of these neurons [38-42]. Altered morphology, number and function of VENs in autism spectrum disorder [43] propose their role in complex social-emotional functions, and complicated cognition such as selfawareness, judgment and emotions. Thus, the emergence of VENs is of enormous consequence in human brain evolution.
Recently ubiquitous localization of VENs in many neocortical regions of pygmy hippopotamus and their presence in layer II of the frontopolar cortex.
Artiodactyls especially domesticated pig, sheep, cow, and whitetailed deer [34] also abandoned the speculation that these neurons are the specialization of only hominids and large mammals.
Taken together, these interpretations imply that the VENs functions have been associated with their presence in functionally distinct areas of the human brain. But the presence of these cells in the diverse group of animals emphasized the need to understand the function of these cells in different animals.
The number and arrangement of VENs have also been found to be proportionately related to the complexity of social interactions of the hominid species. Socially humans and bonobos are the most interactive in which VENs are almost clustered and numerically high. The number is the highest in humans than in gorillas, and least in orangutans which had only a few isolated VENs [23]. On the basis of the review, we have reason to speculate that vocal production learning is the common function in all VENs containing animals and these neurons may also be present in those bird taxa that are known to produce phonological syntax naturally. Parrots’ oratory skill and their emotional make-up compel to think, that their neuronal components may provide some special clues, to understand the process of evolution of vocal production learning and language. Until now, it is also not clear what type of neuroarchitecture is responsible for such complex cognitive behavior and social intelligence in birds, while the high brain- body ratio, brain circuitry and connectivity of birds and mammals are found remarkably similar [19,44]. Comparative study of neural groupings characteristic of bird telencephalon and the laminar arrangement of neurons found in the mammalian neocortex is necessary to determine the structural homologous relationship between bird pallium and mammalian neocortex.
The present paper is an attempt to explore in much greater detail the possible shared neural features of birds and mammals that may generate such higher levels of consciousness, and also determine whether a morphologically similar neuroarchitecture is present in parrots which display complex intelligent behavior similar to intelligent mammals. This study also explicates that the large spindle neurons which are unique to all those brains that are thought to be responsible for social organization, empathy, speech, and emotion, may also be present even in non-mammalian convergently related groups with equivalent behavior competence.
Materials and Methods
An approximate ten-year-old parrot, remarkably capable of imitating human voice was tamed and caged for behavioral studies for two years. This parrot died naturally and the brain had been dissected out and immediately fixed in 4% Paraformaldehyde in 0.1m phosphate buffer (4°C, pH 7.4) for four days. Two budgerigars (Melopsittacus undulatus) approximately five years old were purchased from local bird market of Allahabad (U. P), India. A combination of Ketamine (24 mg/Kg) and Xylazine (12mg/Kg) was injected through the vein of left leg to anesthetize. Animals initially perfused intracardially with physiological saline (0.9% NaCl) at 4°C and then with fixative solution (2% Paraformaldehyde in 0.1m phosphate buffer 4°C, pH 7.4). Two adult chickens (Gallus gallus) had also been purchased. Their brains were removed quickly and kept in the same fixative solution overnight.
Brains of all the specimens were cut along the sagittal plane and divided into two hemispheres.
Golgi staining
After fixation the left hemispheres of telencephalon of all the specimens were incubated in a chromating solution of 2% potassium dichromate and 5% glutaraldehyde for approximately 3 days (Golgi- Colonnier method). Metal impregnation was then done in 0.75% silver nitrate solution for 24–36 hours. After the first round of staining, the chromation and silver impregnation steps were repeated twice for two days, with gradually increasing the length of the silvering time and reduction of chroming time. In all the cases tissues were washed in distilled water between changes in solutions and fixative was replaced with a freshly prepared solution. Approximately 25 ml of solution was used for each brain and kept in the dark. After the completion of third and final impregnation all the pieces were dehydrated for two to five minutes immersions in increasing concentrations of alcohol, and then in xylene for 5 min. Finally, hemispheres were immediately embedded in paraffin at 37°C and sectioned at 100 μm on a sliding microtome. Sections were then proceeded to remove paraffin by treating with xylene and 100% ethanol. Sections were then mounted on clean slides and cover-slipped using DPX.
Nissl stain
Paraffin blocks of the right hemisphere of all specimens were prepared, 20 μm serial sections were cut by sliding microtome and sections were hydrated through a cold decreasing ethanol series and incubated for approximately 5 minutes in a filtered 0.5% cresyl violet (Sigma) solution prepared in acetate buffer (0.1 M, pH 3.5). The sections were differentiated and dehydrated in 95% and 100% ethanol, cleared in the xylene substitute and cover slipped with DPX.
Sections were studied and photographed by using a digital photo automat camera (Nikon). Single neurons were traced, and drawings of neurons were prepared with a camera Lucida. Serial sections from both hemispheres of the brain were studied for detailed morphological analysis of Golgi-impregnated and Nissl stained neurons.
The vocal control nuclei in parrot, has been identified with the help of budgerigar brain atlas in which, these vocal areas are previously identified by a combination of methods, which includes tract tracing, Nissl staining of brain sections, lesioning of specific brain regions, electrophysiological recordings [45-47] and vocalizing driven gene (ZENK) expression [48-49]. Boundaries of all nine vocal control nuclei, as well as other nuclei, have been identified and traced in each Nissl stained series sections. All the telencephalic, thalamic and mesencephalic nuclei were clearly demarcated due to staining of various degrees. Morphologically distinct neuronal classes present in vocal counterparts have also been studied by Golgi-impregnation method. The neurons which were well impregnated were used to study the detailed morphological analysis. Morphometry and scales shown in Photomicrographs and drawings were determined by ocular scale calibrated for each objective using a stage micrometer.
Statistical analysis
We performed Sholl analysis [50] for Morphometry using ‘simple Neurite tracer’ Image J (Fiji) to characterize the morphologic neuronal class of imaged neuron. The Initial quantification of a neuron is performed by counting the number of dendritic intersections for concentric circles, usually centered at the center of soma, of the gradually increasing Sholl radius. Using Sholl analysis we selected variables that contributed most to the overall variability to distinguish different neuronal types.
Terminology and abbreviations are used according to revised nomenclature for telencephalon and some related brainstem nuclei [51,52].
Results
Parrot brain is the largest sized brain among birds and has a noticeable notch between both large cerebral hemispheres that isolates the telencephalon into two halves. In the dorsal view, telencephalon is narrowed at the rostral side (Figures 1A, B, and C). Indian parrot the- Psittacula krameri are characterized by high EQ values that suggest high brain volumes in relation to their body masses; parrot brains are structured differently than the brains of other vocal learning birds, likesongbirds and hummingbirds. Here detailed anatomical investigation of the brain of Indian parrot–reveal that its neuroarchitecture and brain wiring is unique with specialized cells and without multilayered cortical structure. All the telencephalic, thalamic and mesencephalic areas show the varied staining intensity in Nissl stain and thus are clearly demarcated. Figures 1A-D represents the dorsal, ventral, lateral view of parrot brain. The vertical lines in (Figure 1E) indicate the locations of the sections of interest. The outlines of different nuclear parts of green ring neck Parrot were found to be almost same when compared with budgerigar vocal areas. Outline of each of these areas has been prepared by using camera Lucida (Figures 2A–F). Detailed examination of Nissl and Golgi stained transverse series sections of brain of two species of parrot unveiled the presence of specialized class of neurons in several locations in lateral medial regions of telencephalon, particularly, in Nidopallium Caudolaterale (NCL), dorsolateral corticoid region (CDL), Arcopallium (A) and their corresponding vocal nuclei. All these areas were identified on the basis of the Modern consensus view of the avian brain as described by Jarvis [53] (Figure 2A-F). These regions accomplish sensory processing, motor control and sensory-motor learning in birds similar to the mammalian neocortex. In these brain regions, we discovered the presence of two unique cell morphotype comparable to Von Economo Neurons (VENs) and fork cells in humans, great apes, [23,36,24], and macaque monkey [30]. The neurons which share morphological similarities described by the classical neuroanatomists such as Von Economo [53] in humans, classified as VENs on the basis of the presence of a large elongated soma, a prominent basal and apical dendrite and presence of the nearest complete pyramidal neurons [23] and fork neurons [30]. VENs were identified as large, rod and corkscrew, atypical pyramidal type, bipolar, projection neurons- characterized with elongated, spindle- shaped perikaryon, large apical dendrite, and a single basal dendrite with very sparse branching (Figures 3A and B). Fork cells are characterized by bifurcated long apical dendrites and a single basal dendrite, (Figure 3G) analogous to the fork cells found in frontoinsular, the cortex of humans, great apes [54] and macaque monkey [30]. Both are exceptional neuronal type present intermingled with each other and were larger than neighboring pyramidal cells (Figures 3E-G) and noticeably larger than surrounding fusiform neurons (Figures 3I & 4C, D). Figure 3-A, B illustrate the magnifying view of VENs and (Figure C) demonstrate magnified soma of a VEN. Specific alteration of both these neuronal type in humans is noticed in behavior variant frontotemporal dementia [39,42] and associated with social awareness, empathy, and control of appetite [39]. Lateral part of Oval nucleus of anterior nidopallium (NAOc) and the central Nucleus of the Lateral nidopallium (NLc), (Figures 2A-D) contributes to, the Nidopallium Caudolaterale (NCL) region - a callopallial anterior region of the telencephalon. NCL was located posterior- laterally in nidopallium towards the lateral side of arcopallium (Figures 1B, 2D and 4A). In this nucleus, the cells were arranged in both radial and vertical fashion and can be distinguished as large spindle-shaped bipolar neurons (VENs) Pyramidal neurons (PY), and Fork neurons (F) (Figure 4C). VENs and fork cells are also predominantly confined to another well-demarcated nucleus in parrot telencephalon- the AAC (Figure 4D) - located towards the caudomedial side of NLC (Figures 1E, 2D, 4A, and 4B) covers the rostral part of arcopallium (Figures 2D and 4A). These cells were more dispersed in AAC in comparison to NCL (Figure 4D).
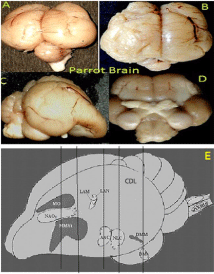
Figure 1: (A- D) Represents the macroscopic views of the brain of the parrot
investigated in the present study. Dorsal (A,B) ventral (D) and lateral (C).
(E) –diagrammatic representation of the brain of Indian ring neck parrot -
demonstrate locations of all nine vocal control areas in caudolaterale
telencephalon proposed by Jarvis & Mello 2000.
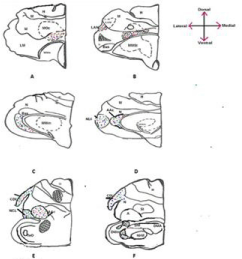
Figure 2: Represents, Camera Lucida drawing prepared from Nissl stained
sections of parrot brain. Locations and outlines of all vocal counterparts and
other nuclei are demarcated in (A-F). Red, blue and green colored dots in
different areas indicate the distribution of VENs, fork and pyramidal cells
respectively.
Figures 5A and B represents the distribution of VENs in CDL region. CDL exhibits the lower density of VENs in comparison to arcopallium region. However, the number of VENs increased from rostral to caudal region of CDL. It has also been noticed that spindle cells measured in this area had larger soma size, greater extension of the dendritic field and were occasionally present in clusters or group of 3 to 4 neurons (Figures 3D, E and 6A). VENs are demonstrated in higher magnification in (Figures 3D and E). However fork cells have not been observed in this area, only a few pyramidal neurons found to be present infrequently intermingled with VENs.
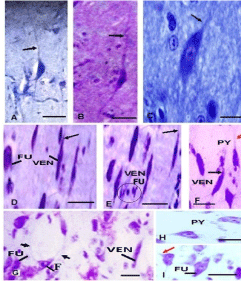
Figure 3: (A,B) High-magnification photomicrographs of Nissl stained
sections demonstrating the different morphology of the parrot VENs identical
to macaque, cetaceans, and human. (A)-VEN in Budgerigar, (B)- VEN in
parrot (C) - Magnified stout spindle-shaped soma with single apical and
single basal dendrite of VEN showing nucleolus. (D & E) - VENs in high
magnification in CDL area with extremely slender cell body as noticed in
hominid (Nimchinsky et al.1995) and cetaceans, (Butti et al. 2014). (F)-
Magnified image of VEN and Pyramidal cell in Arcopallium. (G)- Demonstrate
the fork cell in Nissl stained sections with bifid soma and two apical dendrites,
long black arrow in all photographs indicates the apical dendrites of VENs,
red arrow indicates apical dendrite of pyramidal cells and short arrows
indicate the apical dendrites of fork neurons. Circle in (Figure E) indicate the
small fusiform cells. (H)- Single pyramidal cell – (I) - single large fusiform cells
in high magnification. -scales =50 μm in all photograph.
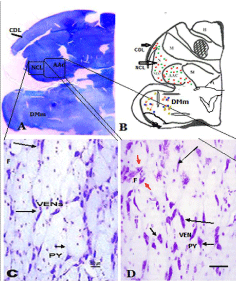
Figure 4: (A) Scanned micro photomicrographs of the Nissl stained
coronal section of caudal telencephalon of parrot showing the location
and arrangement of the large bipolar neuronal types in NLC and AAc. The
insert shows the distribution of VENs and fork neurons in NCL and AAc.
(B)- Camera Lucida drawing of the same section of parrot brain indicates
the distribution of VEN, PY, and fork cells in AAC and NLC and CDL region.
(C)- Photomicrograph of Nissl stained section showing a mixed population of
VENs, Forks and Pyramidal neuron (PY) in NCL. (D)- Distribution of neurons
in AAc. The long black arrow indicates VENs and the short black arrow
indicates PY cells. Red Arrows indicates fork neurons.
Additionally, the presence of bipolar VENs, Fork cells and pyramidal neurons has been noticed in Magnocellular nucleus of the dorsal thalamus (DMm) identified and named by Striedter [45], as a region in the dorsal thalamus demarcated by larger cells than the surrounding areas (Figures 5A, C, D and 6C). These cells are sparsely distributed in this area. A small concentration of VENs and comparatively smaller pyramidal neurons were also observed at the lateral and dorsal part of DMm. On the anterior- medial side of DMm, a small area demarcated as the Dorsomedial nucleus (DM) of Inter Collicular complex (ICo) were also found having some large bipolar neurons. Cells of the Dorsal Medial nucleus of the midbrain (DM) were in low density and were found scattered in the nucleus. In addition to VENs, Pyramidal and fork neuron another unique neuronal type-the enveloping neuron, (Figure 6D) and small to large multipolar and smaller fusiform cells have also been investigated in this study. Surprisingly, all these types are mostly present in humans and nonhuman mammals and have not been noticed ever in any bird species. Fork and enveloping neurons have been previously described in the human insular cortex by Ngowyang [54]. We recognized “enveloping neurons” as a type that was commonly observed adjoining capillaries or other cells as previously described by de Crinis [55], Syring [56] et al. Either their soma or dendrite would “cuddle” the space created by a capillary [57] (Figure 6D). Figure 6E indicates the magnified view of fork cells in DMm. For comparative purposes, we also examined the brain of adult chicken, the close relative of parrot as a representative of the vocal non-learner bird. However, VENs and Fork neurons types have not been observed in any brain areas of Nissl stained sections of the chicken brain.
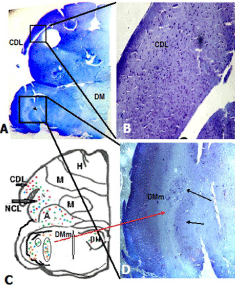
Figure 5: (A) Scanned micro photomicrographs of the Nissl stained coronal
section of caudal telencephalon of parrot showing the location of the presence
of large bipolar neuronal types in CDL and DMm regions. The insert tracing
shows the magnified view of area CDL and DMm showing the arrangement
of large bipolar neurons. (B)-camera Lucida drawing of the same section
specifies location of the nuclei. Red, blue and green colored dots indicate the
distribution of VENs, fork and pyramidal cells respectively. Arrows indicate
the location of presence of VENs, and fork cell type.
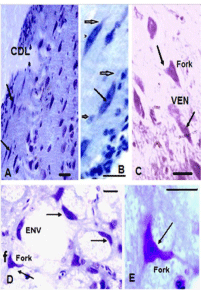
Figure 6: (A) Photomicrographs of Nissl stained sections through dorsolateral
corticoid (CDL) area of parrot displayed the morphological characteristics
and location of bipolar neurons. (B) – Magnified view of VENs in CDL. (C)-
Photomicrograph of magnifying view of VENs and fork cells in mesencephalic
nuclei, DMm. (D)- Distribution of, Enveloping neurons (ENV), and fork
neurons (F) in DM. Scale = 50μm in all photograph.
In Golgi stained sections, distinctive morphology, unusual size, characteristic stout, elongated, gradually tapering, spindle soma which virtually remains symmetric in its vertical and horizontal axis having distantly-branched long apical dendrite and a basal dendrite, strongly verify the correspond of these cells to mammalian VENs (Figures 7A and B). The basal dendrite also distantly branched into spiny dendrites. Golgi stained Fork cells are similar to VENs in their basal aspect with a single large tapered dendrite, but they are distinguished by two long apical dendrites that bifurcate close to the soma and appear in a Y-like fashion. They represent the morphological characteristics of pyramidal projection neurons (Figures 7A and 8A,B). Figure 8C represents the dendritic extension of VENs and fork neurons. Cells that formed another subgroup in most of the regions where VENs were present, could be characterized as tuft-less pyramidal or at least pyramidal-like, based on the presence of conical cell bodies, distinct dendrite emanating from the apical part of the soma and an array of smaller basal dendrites [24] (Figures 8 D,E,F). Figures 9A-D represents Camera Lucida drawing of VENs and different types of Pyramidal like (PY) neurons present in various regions.
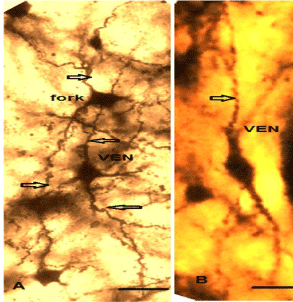
Figure 7: (A) Golgi stain parrot VENs possesses the signature feature of
pyramidal projection neurons of the human VENs, long apical dendrite and
a single long occasionally sparsely branched basal dendrite (Nimchinsky et
al.1995) a fork neurons also present intermingled with VEN characterized
by two thick apical dendrites. Dendritic spines are sparse in both types of
neurons. Arrows point out the apical and basal dendrite. (B)- Single VEN with
the extension of the apical and basal dendrite.
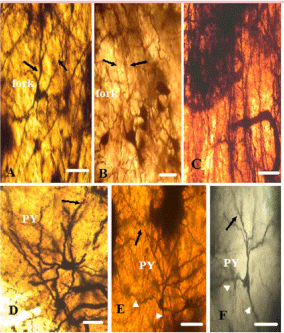
Figure 8: Photomicrographs of Golgi stained fork and Pyramidal cells. Scale
bar same for all figures. Scale bar- 50 μm. (A&B): indicates long bifurcated
apical dendrites of fork neuron projecting towards gray matter. (C): dendritic
extension of VENs and fork neurons. (D, E, F): Single typical pyramidal
neuron with single apical dendrite indicated by black arrow. The short white
arrow indicates branched basal tuft closed to soma. Primary and secondary
dendrites possess the significant number of spines. Apical dendrite branched
near soma. Axon arises from the base of soma and branched.
Neurite tracer (Fiji) was used to create Sholl radii, for 20 VENs in nidopallium regions and 12 VENs of arcopallium and CDL region. Sum of all Sholl radii indicate that total length and intersections number of apical and basal dendrites of VENs and fork was remarkably similar. The number of dendritic Spines per 10 μm along the extent of all dendrites on the apical dendrite was highest up to 250 to 280 μm, ± 51.87 and for the basal dendrite; it was 260 to 280 μm ± 54.57 μm (P= .001). The Soma of this cell type was exclusively large about 70 ± 6.74 to 80 ± 4.81 μm in length and 25 ± 5.67 to 30 ± 7.82 μm in width. The apical dendritic field extension was exclusively long. The total dendritic size varies from 711.5624 ± 43.57 μm (P= .001). The max Sholl radii for apical dendrite were 230 ± 37μm and 220 ±17.89 μm for the basal dendritic tree (P= .001). However, in NLC region basal dendritic field of a few VENs is relatively much shorter, about 70 ± 26.76 – 90 ± 78.67 μm in budgerigar (Figure 9D). The apical dendrite of PY neurons gave rise to several branches and in a few cases bifurcated at 50–100 μm from the cell body (Figures 8C and D). The somatic roundness was 05.57 ± 0.21, n= 07. We also found another group of so-called small pyramidal type neurons with conical cell bodies, a distinct whip like long apical dendrite and sparsely branched basal skirt (Figures 8C and D). The common feature of cells in this group was the lack of an apical tuft. Both groups of PY cells included cells that were often atypically oriented, i.e., the cell body and the dendritic tree as a whole extend diagonally, instead of typically perpendicularly, somatic roundness was (0.75 ± 0.08, n= 08). The average dendritic length was 189μm ± 79.9; the dendritic arbors cover a significantly larger region around the soma. On the basis of dendritic branching, dendritic length and the number of spines (intersections numbers) calculated by Sholl analysis we analyzed that; the dendritic complexity of apical dendrite of VENs and fork type neurons was approximately similar. The average critical value of apical dendrites of VENs is 280.369 and 278.693 for fork neurons. Apical and basal dendrites of VENs and fork cells were found symmetric in terms of intersections numbers. Figures 10A-D demonstrates Comparative dendritic architecture of VENs, fork and pyramidal cells of parrot and in budgerigar. However, the basal dendritic skirt of pyramidallike neurons shows higher Sholl intersections, having average critical value 390.8247 ± 108.78. The Sholl regression coefficient (the measure of change in density of dendrites as a function of distance from the cell body) of VENs is .oo3 to.oo4 and 2.5 to 9.5 for the pyramidal neuron. From the above results, it can be concluded that the basal tufts of pyramidal-like neurons contain more Sholl intersections than apical dendrites.
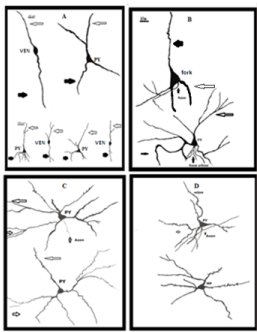
Figure 9: Represents Camera Lucida drawing of VENs, fork and different
types of Pyramidal (PY) neurons, present in various regions. Scale bar same
for all drawings. Long arrows point out the apical dendrites, thick arrows
indicate the basal dendrites, and very short arrows represent axons of VENs,
fork and Pyramidal neurons.
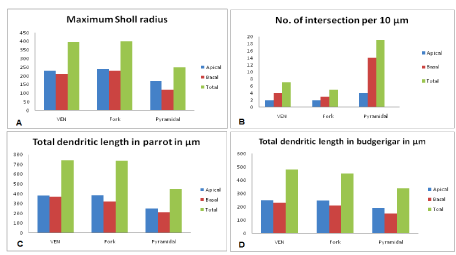
Figure 10: (A-D) Represents quantitative analysis of dendritic morphology
of VENs, fork and Pyramidal neurons- Comparison of dendritic structure
for apical and basal dendritic tree of VENs, fork and pyramidal neurons of
CDL, NCL and Arcopallium region for (A) maximum scholl radii (B) no of
intersections/ 10 μm (C) total dendritic length in μm. results indicate that VENs
and fork cells have comparatively fewer intersections and sparsely spinous.
There is no any significant difference in total dendritic length of apical and,
the basal dendrite of VENs and fork neurons, however, pyramidal neurons
are smaller than VENs and fork cells and more spinous (more intersection
number). (D)-Total dendritic length of the apical and basal dendrite of VENs,
fork and pyramidal neurons in budgerigar is smaller than that of parrot.
Discussion
This study is the first to provide evidence of existence and localization of specialized classes of neurons, especially- pyramidal, VENs, fork neurons, and enveloping neurons in parrot brain; those were exclusively found in V layer of ACC, PFC, and FI of the limited group of socially intelligent mammals. VENs and other exclusive neuronal classes the fork cells and enveloping neurons previously reported only in neocortical regions of humans [54]. Recently VENs and fork cells have also been observed in layer V of ACC, PFC and FI of human, great apes [57,38,58], macaque monkey [30], elephants [59], and cetaceans [27] and in the putative visual cortex of pygmy hippopotamus [32]. Our results demonstrate that such neuronal morphology is also present in bird species which do not have a laminated multilayered cerebral cortex like mammals but represent the higher level of cognition. It is very interesting to note that VENs were found only in vocal learner mammals. Vocal learning [60], the highest form of social learning, incorporate the capability to modify auditory and syntactic sounds, or reproduce the new combinations of sounds, is a rare trait, and a critical substrate for spoken language, predominantly observed in only limited species particularly humans, cetaceans [1], elephants [3], seals [2,4,5], Pinnipeds (walrus) [6], Non-Human Primates [61] and Japanese and rhesus monkeys [61]. These cells were not observed in any other primate species or any mammalian taxa those do not have vocal acquisition and social cognition, indicate their involvement in the production of vocalizations and communicative skills. We have also not found VENs in any brain area of vocal non-learner birds like chicken, which also indicates the correlation of VENs with vocal acquisition. The non-human great apes and Orangutans are unable to mimic the sound, but have a complex system of communication (sign language) and have VENs. Remarkably, in the cerebrum of all three vocal learning bird groups i.e songbird, hummingbird, and parrot, there are seven comparable vocal brain nuclei: four posteriorly located nuclei - NLc (In Nidopallium), AAc (in Arcopallium), DM and medulla (nXIIts), forming a posterior vocal pathway that project from NLc to AAc, to DM and medulla vocal motor neurons (nXIIts) [45,46,62,63] nXIIts projects to the muscles of the syrinx, the avian vocal organ. This pathway is responsible for the production of learned vocalizations. Vocal non-learning birds have DM and nXIIts, but not having projections from the arcopallium [64] for the production of innate vocalizations. Jarvis [20] proposed that humans also have posterior and anterior vocal pathways for production and learning of language. The human posterior vocal pathway which consists of the face motor cortex and its projections to midbrain the Periaqueductal Gray (PAG) and brainstem the nucleus Ambiguous (Am) motor neurons which are responsible for the production of speech. Like in vocal non-learning birds, the (PAG) and (Am) in vocal non-learning mammals do not receive motor cortical (pallial) projections and they control the production of innate sounds [65-67]. In this manner, Jarvis [68] argued that a mutational event that caused descending projections of avian arcopallium neurons to synapse onto nXIIts or mammalian layer 5 neurons of the face motor cortex to synapse onto may be the only independent major change that is needed to initiate a vocal learning pathway. We found projection neurons (VENs and fork), in vocal control nuclei of Arcopallium (AAC) of Indian parrot, which may involve in the circuitry of posterior vocal pathway responsible for vocal acquisition in parrot, similar to layer V neurons of vocal learner mammals. It might signal the further anatomic and possibly functional amplification of this area in the mammalian lineage known to have evolved verbal communication and its emotional implications. Thereafter, other vocal brain regions might have developed out of contiguous motor brain regions dependent on pre - accessible connectivity [69]. The proposed human anterior vocal pathway consists of a loop [69,20] that includes projections from a lateral-to-medial strip of premotor cortex which consists of the anterior insula, Broca’s area, the anterior dorsal lateral prefrontal cortex, the pre-supplementary motor area, and the anterior cingulate cortex. Most of these areas are VENs containing regions in humans and vocal learner mammals. This strip projects on to an anterior region of the striatum, to the globus pallidus, to an anterior portion of the dorsal thalamus, and then back to the cortex. Similarly, in parrots, three anteriorly located nuclei NAOc, MMSt, and DMM (VENs containing regions of parrot) are part of an anterior vocal pathway [20] where a pallial vocal nucleus (NAOc) projects to the striatal vocal nucleus (MMSt) the which projects to a nucleus of the Dorsal thalamus (DMm), and the dorsal thalamus projects back to the pallial vocal nucleus [46,62]. Lateral part of three major Nidopallium vocal nucleus including NAOc, NLc and LAN also contributes to NCL region (the nidopallium caudolaterale) a callopallial anterior region, already proposed as homologous to mammalian prefrontal cortex PFC [70-74]. Recent evidence suggests that avian NCL is functionally equivalent to the mammalian PFC and is involved in higher cognitive functions like numerical understanding [18,20], working memory [75], and insightful behavior [74]. Additional evidence for the functional equivalence of the avian nidopallium and mammalian prefrontal cortex comes from studies of the distribution of dopamine in the avian telencephalon [76-77], which is also widely distributed in the mammalian frontal and prefrontal cortex [78]. The presence of VENs in NCL area of parrot suggests that complex cognitive functions of birds are due to similar specialized neuronal components of homologous regions of the brain which forms the neural circuitry responsible for cognition and vocal learning.
The lemnopallial region called the CDL [79], most recently, has proposed as homologous to mammalian cingulate cortex [80]. Lesions in the anterior cingulate cortex in human are associated with a form of mutism [81-83]. It is one of the only cortical areas known to elicit meaningful vocalizations (and not merely sounds) in squirrel monkeys when stimulated [83]. In the monkey, the ventral anterior insula has been associated with viscerosensory and -motor functions including vocalization [84-86]. Thus, this region may be involved in some aspects of communication in primates, and this is the area to which the VENs are restricted. The existence of VENs in dorsal lateral corticoid area (CDL) of parrot brain also shows the cytoarchitectural equivalence of this area with primates.
Conclusion
It is possible that the appearance of the combination of unique neuronal types -VENs and fork cells in some pallial regions of a few bird species may be the first step in the evolution of social behavior and vocalization. The structural arguments of neural substrate imply that lineage close to human, like apes, cetaceans, and elephants and now parrots and may be some other birds have the parallel morphological neural substrate to maintain rapid transmission of crucial information. All these orders appear to have arisen independently and developed large brain size and specialized large spindle neurons responsible for their social behavior, and vocal acquisition.
Acknowledgement
This research work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We thank Prof. U.C. Srivastava (Professor in Neurobiology, Dept of Zoology, University of Allahabad U.P. India) for his valuable suggestions and allowing me to use his laboratory space to perform photography by the digital Photo-automat camera (Nikon eclipse- Boi). We thank to Dr. Vinay Khanna, Principal Scientist at IITR, Lucknow, U P, India, for his very useful assistance in photography of neurons.
References
- Janik VM, Slater PJB. Vocal learning in mammals. Adv Study behaves. 1997; 26: 59-99.
- Sanvito S, Galimberti F, Miller EH. Observational evidences of vocal learning in southern elephant Seals, A longitudinal study. Ethology. 2007; 113: 137-146.
- Poole JH, Tyack PL, Stoeger-Horwth, AS, Watwood S. Animal behavior. Elephants are capable of vocal learning. Nature. 2005; 434: 455-456.
- Ralls K, Fiorelli P, Gish S. Vocalizations and vocal mimicry in captive harbour seals, Phoca vitulina. Canadian Journal of Zoology. 1985; 63: 1050–1056.
- Lilly JC. Vocal mimicry in tursiops: ability to match numbers and durations of human vocal bursts. Science. 1965; 147: 300–301.
- Schusterman RJ, Reichmuth C. Novel sound production through contingency learning in the Pacific walrus (Odobenus rosmarus divergens). Anim Cogn. 2008; 11: 319-327.
- Reynolds JE, Odell KO. Manatees and Dugongs Facts on File, Inc., New York, USA. 1991.
- Kittler K, Schnoell AV, Fichtel C. Cognition in Ring-Tailed Lemurs Cognition in Ring-Tailed Lemurs. Folia Primatol. 2015; 86: 106–116.
- Weary DM, Fraser D. Vocal response of piglets to weaning: effect of piglet age. Applied Ani Behav Sci. 1995; 54: 153-160.
- Michael CA, Daniel MW, Allison AT, Gudrun I. Vocal Communication in Pigs: Who is Nursing Piglets Screaming at? Ethology. 1999; 105: 881-892.
- Bauernfeind AL, de Sousa AA, Avasthi T, Dobson SD, Raghanti MA, Lewandowski AH, et al. A volumetric comparison of the insular cortex and its subregions in primates. J Hum. 2013; 64: 263-279.
- Coudé G, Ferrari PF, Rodà F, Maranesi M, Borelli E, Veroni V, et al. Neurons Controlling Voluntary Vocalization in the Macaque Ventral Premotor Cortex. PLoS ONE. 2011; 6: e26822.
- Kershenbaum A, Ilany A, Blaustein L, Geffen E. Syntactic structure and geographical dialects in the songs of male rock hyraxes. Proceedings of the Royal Society. 2012; 1–8.
- Briefer E, Vannoni E, McElligott AG. Quality prevails over identity in the sexually selected vocalizations of an ageing mammal. BMC Biology. 2010; 8: 35.
- Padilla M Briefer EF, Reader T, McElligott AG. Acoustic analysis of cattle (Bos taurus) mother–offspring contact calls from a source–filter theory perspective, applied. Ani Behav Sci. 2015; 163: 58-68.
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005; 3: e386.
- Knörnschild M, Nagy M, Metz M, Mayer F, Helversen OV. Complex vocal imitation during ontogeny in a bat Biology Lett. 2010; 6: 156-160.
- Pepperberg I. The Alex Studies: Cognitive and Communicative Abilities of Grey Parrots. 1999; 96-167.
- Butler AB, Cotterill RMJ. Mammalian & Avian Neuroanatomy and the question of consciousness in birds. Biol Bull. 2006; 211: 106-127.
- Jarvis ED. learned bird song and the neurobiology of human language. Ann NY Acad sci. 2004; 016: 749-777.
- Kroodsm ADE. Reproductive development in a female songbird: differential stimulation by quality of male song. Science. 1976; 192: 574-575.
- Sudhi S. Emotion, cognition, social intelligence and related brain network In Indian green ring neck parrot the Psittacula krameri accepted in. J Exp Zool India. 2011; l: 15.
- Nimchinsky EA, Gilissen E, Allman J, Perl D, Erwin J, Hof P. A neuronal orphologic type unique to humans and great apes. Proc Nat Acad Sci USA. 1999; 96: 5268–5273.
- Watson KK, Jones TK, Allman JM. Dendritic architecture of the von Economo neurons Neuroscience. 2006; 141: 1107–1112.
- Fajardo C, Escobar MI, Buriticá E, Arteaga G, Umbarila J, Casanova MF, et al. Von Economo neurons are present in the dorsolateral (dysgranular) prefrontal cortex of humans. Neurosci Lett. 2008; 25: 215-218.
- Allman JM, Tetreault NA, Hakeem AY, Park S. The von Economo neurons in apes and humans. American journal of human Biology. 2011; 23: 5-21.
- Hof PR, Gucht VD. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae). Anat Rec (Hoboken). 2007; 290: 1-31.
- Butti C, Sherwood CC, Hakeem, AY, Allman JM, Hof PR. Total number and volume of Von Economo neurons in the cerebral cortex of cetaceans. J Com Neurol. 2009; 515: 243-259.
- Hakeem AY, Sherwood CC, Bonar CJ, Butti C, Hof PR, Allman JM. Von Economo neurons in the elephant brain. 2009; 292: 242-248.
- Evrard HC, Forro T, Logothetis NK. Von Economo neurons in the interior insula of the macaque monkey. Neuron. 2012; 74: 482-489.
- Butti C, Santos M, Uppal N, Hof PR. Von Economo neurons: clinical and evolutionary perspectives Cortex. 2013; 49: 312-326.
- Butti. The Cerebral Cortex of the Pygmy Hippopotamus, Hexaprotodon liberiensis (Cetartiodactyla, Hippopotamidae): MRI, Cytoarchitecture, and Neuronal Morphology. Anat Rec. 2014; 297: 670–700.
- Raghanti MA, Spurlock LB, Treichler FR, Weigel SE, Stimmelmayr R, Butti C, et al. An analysis of von Economo neurons in the cerebral cortex of cetaceans, artiodactyls, and perissodactyls. Brain Struct Funct. 2015; 220: 2303-2314.
- Rose M. Die Inselrinde des Menschen und der Tiere. J Psychol Neurol. 1928; 467–624.
- Butti C, Hof PR. The insular cortex: a comparative perspective. Brain Struct Funct. 2010; 14: 477.
- Allman JM, Watson K, Tetreault N, Hakeem A. Intuition and autism: A possible role for Von Economo neurons Trends cogn sci. 2005; 9: 367-373.
- Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, Dearmond SJ. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006; 60: 660–667.
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, et al. The Von-Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010; 214: 495-517.
- Seeley WW, Allman JM, Carlin DA, Crawford RK, Macedo MN, Greicius MD, et al. Divergent social functioning in behavioral variant fronto-temporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis Assoc Disord. 2007; 21: 50–57.
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function Brain. Struct Funct. 2010; 214: 655-667.
- Cauda F, Costa T, Palermo S, D’Agata F, Diano M, Bianco F, et al. Concordance of white matter and gray matter abnormalities in autism spectrum disorders: a voxel- based meta-analysis study. Hum Brain Mapp. 2014; 35: 2073–2098.
- Kim EJ, Sidhu M, Gaus SE, Eric J, Hof PR, Miller BL, et al. Selective Fronto-insular von Economo Neuron and Fork Cell Loss in Early behavioural Variant Frontotemporal Dementia. Cereb Cortex. 2012; 22: 251-259.
- Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, et al. Von Economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Res. 2011; 1380: 206–217.
- Gu¨ntu¨rku¨n O. The convergent evolution of neural substrates for cognition Psychological Research. 2012; 76: 212–219.
- Striedter GF. The vocal control pathways in budgerigars differ from those in songbirds. J Comp Neurol. 1994; 343: 35–56.
- Durand SE, Heaton JT, Amateau SK, Brauth SE. Vocal control pathways through the anterior forebrain of a parrot (Melopsittacus undulatus). J Comp Neurol. 1997; 377: 179-206.
- Nottebohm F, Stokes TM, Leonard CM. Central control of the song in the canary Serinus canaria. J Comp Neurol. 1976; 165: 457-486.
- Jarvis ED, Ribeiro S, da Silva ML, Ventura D, Vielliard J, Mello CV. Behaviorally driven gene expression reveals song nuclei in hummingbird brain. Nature. 2000; 406: 628–632.
- Jarvis ED, Mello CV. Molecular mapping of brain areas involved in parrot vocal communication. J Comp Neurol. 2000; 419: 1–31.
- Sholl DA. Dendritic organisation in the neurons of the visual and motor cortices of the cat. J Anat. 1953; 87: 387-406.
- Reiner A. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J comp Neurol. 2004; 473: E1-E6.
- Jarvis ED. Selection for and against vocal learning in birds and mammals. Ornithol Sci. 2006; 5: 5–14.
- Von Economo C. The Cytoarchitectonics of the Human Cerebral Cortex. Oxford University Press. Oxford England. 1929.
- Ngowyang G. Beshreibungeiner art von specialzellen in derinselrinde. J Pyshol Neurol. 1932; 44: 671- 674.
- de Crinis M, Über. Die Spezialzellen in der menschlichen Grosshirnrinde. J Psych Neurol. 1934; 45: 439- 449.
- Syring A. Die verbreitung von Spezialzellen in der grosshirnrinde verschiedener säugetiergruppen Cell and Tissue Research. 1957; 45: 399–434.
- Stimpson CD, Tetre, Ault NA, Allman JM, Jacobs B, Butti C, et al. Biochemical specificity of von Economo neurons in hominoids. Am J Hum Biol. 2011; 23: 22-28.
- Seeley FT, Merkle SE, Gaus AD, Craig JM, Allman PR, Hof CV. Economo Distinctive neurons of the anterior cingulate and frontoinsular cortex: a historical perspective Cereb. Cortex. 2012; 245-250.
- Jacobs B, Lubs J, Hannan M, Anderson K, Butti C, Sherwood CC, et al. Neuronal morphology in the African elephant (Loxodonta Africana). Brain Struct Funct. 2011; 215: 273-298.
- Snowdon CT. "Plasticity of communication in non-human primates”. 2009; 239–276.
- Masataka N, Fujita K. Vocal learning of Japanese and rhesus monkeys. Behaviour. 1989; 109: 191–199.
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo-“cortical” loops in the song system of oscine songbirds. J Comp Neurol. 1997; 380: 275–290.
- Gahr M. Neural song control system of hummingbirds: comparison to swifts, vocal learning (Songbirds) and nonearning (Sub oscines) passerines, and vocal learning (Budgerigars) and nonlearning (Dove, owl, gull, quail,chicken) non passerines. J Comp Neurol. 2000; 426: 182–196.
- Wild JM, Li D, Eagleton C. Projections of the dorsomedial nucleus of the intercollicular complex (DM) in relation to respiratory-vocal nuclei in the brainstem of pigeon (Columba livia) and Zebra Finch (Taeniopygia guttata). J Comp Neurol. 1997; 377: 392–413.
- Kuypers HGJM. Corticobulbar connexions to the pons and lower brain-stem in man. Brain. 1958a; 81: 364–388.
- Kuypers HGJM. Some projections from the peri-central cortex to the pons and lower brain stem in monkey and Chimpanzee. J Comp Neurol. 1958b; 100: 221–255.
- Jurgens. Neural pathways underlying vocal control. Neurosci bio behav Rev. 2002; 6: 235-258.
- Jarvis ED. Selection for and against vocal learning in birds and mammals. Ornithol Sci. 2006; 5: 5–14.
- Lieberman P. On the nature and evolution of the neural bases of human language. Am J Phys Anthropol Suppl. 2002; 35: 36–62.
- Waldmann C, Güntürkün O. The dopaminergic innervations of the pigeon caudolaterale forebrain: immunocytochemical evidence for a “prefrontal cortex” in birds? Brain Res. 1993; 600: 225–234.
- Hartmann B, Güntürkün O. Selective deficits in reversal learning after neostriatum caudolaterale lesions in pigeons: possible behavioral equivalencies to the mammalian prefrontal system. Behav Brain Res. 1998; 96:125–133.
- Diekamp B, Kalt T, Ruhm A, Koch M, Güntürkün O. Impairment in a discrimination reversal task after D1 receptor blockade in the pigeon “prefrontal cortex. Behav Neurosci. 2001; 114: 1145-1155.
- Rose J, Colombo M. Neural correlates of executive control in the avian brain. PLos Biology. 2005; 3: 190.
- Kirsch JA, Güntürkün O, Rose J. Insight without cortex; Lessons from the avian brain. Consciousness and Cognition. 2008; 17: 475-483.
- Honig WK. Studies of working memory in the pigeon. Cognitive processes in animal behavior. 1978; 211-248.
- Durstewitz D, Kroner S, Gunturkun O. Dopamine innervations of the avian telencephalon. Prog.neurobiol. 1999; 59: 161-175.
- Kubikova L, Wada K, Jarvis ED. Dopamine receptors in a song bird brain. The Jl Comp Neurol. 2010; 518: 741-769.
- Goldman-Rakic PS, Lidow MS, Smiley JF, Williums MS. The anatomy of dopamins in monkey and human prefrontal cortex. J Neurol Trans Suppl. 36: 163-177.
- Montagnese CM, Mezey SE, Csillag A. Efferent connections of the Dorsomedial thalamic nuclei of the domestic chick ( Gallus domesticus). J Comp Neurol. 2003; 459: 301-326.
- Atoji Y, Wild JM. Afferent and efferent connections of the dorsolateral corticoid area and a comparison with connections of the tempero-parietooccipital area in the pigeon (Columba livia). J Comp Neuro. 2005; 485: 165-182.
- Devinsky O, Morrell M, Vogt BA. Contributions of anterior cingulate cortex to behavior Brain. 1999; 118: 279–306.
- Barris RW, Schuman HR. Bilateral anterior cingulate gyrus lesions: syndrome of the anterior cingulate gyri. Neurology. 1953; 3: 44-52.
- Nemeth G, Hegedus K, Nemeth G, Hegedus K, Molnar L. Eur Arch. 1988.
- Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus; a preliminary report. J Neurophysiol. 1949; 12: 347–356.
- Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann NY Acad Sci. 2007; 1121: 54–71.
- Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex Biol. Psychiatry. 2011; 69: 1133–1139.