
Research Article
Austin J Anat. 2018; 5(1): 1080.
Useful Materials for Cross-Sectional Anatomy Education: Silicone Plastinated Examples of Foot and Hand
Bilge O*, Çelik S, Yörük MD and Koçer IB
Department of Anatomy, Faculty of Medicine, Ege University, Turkey
*Corresponding author: Bilge O, Department of Anatomy, Faculty of Medicine, Ege University 35100 Bornova, Izmir, Turkey
Received: February 26, 2018; Accepted: April 05, 2018; Published: April 12, 2018
Abstract
Objective: Cross-sectional anatomy is crucial in comprehension of the whole body. Furthermore, it is mostly encounters the physicians as radiological images during their education or after graduation. The diversity of materials in cross-sectional anatomy education and their ability to match radiological images facilitate the integration of information. Anatomical examples possessing these features can obtain using plastination. We aimed to obtain real, reusable, safe and demonstrative anatomical training materials to facilitate cross-sectional anatomy training. The final products should be thin, semi-flexible, translucent and durable.
Materials and Methods: A specific sheet plastination procedure with a different surface cleaning method is taking place in this study. Foot and hand slices of 2-3 mm were plastinated with xylene added silicone resin to obtain thin, translucent, semi-flexible and durable slices.
Results: The plastinated slices have fine details of many anatomical structures comparable with radiological images. Translucent feature of the sheets provides useful backlit imaging. Sheets are semi-flexible and durable enough to use safely in practices.
Conclusion: Plastination seems to be the ideal method for preparing and preserving cross-sectional specimens. We believe that silicone plastinated sheets are useful materials for laboratories that do not have adequate equipment or experience to make epoxy or polyester sheet plastinates.
Keywords: Cross-sectional anatomy; Sheet plastination; Anatomy education; Silicone
Introduction
Since the invention of plastination technique by von Hagens, it has become the best method for preservation of biological human and animal tissues [1-3]. Plastination has helped to increase the variety of the instructional anatomical specimens by providing dry and odorless condition [4]. Among plastination techniques, sheet plastination is one of the most popular and reformist method for preservation of sliced samples. Due to the rapid developing and varying imaging techniques, cross-sectional anatomy has gained importance in recent years. Sheet plastination ensures detailed exhibition of the internal anatomical structures in their normal positions and provides materials to examine and understand the cross-sectional human anatomy [2,5,6].
Two mostly used resins for sheet plastination are epoxy and polyester. Epoxy resin is preferred to preserve 2-5 mm body slices as semi-transparent and durable sections while polyester resin mostly used for brain sheet plastination [7,8]. P40 polyester technique offers opaque brain specimens but also semi-transparent samples can prepared with P35 and P45 techniques [6,9,10,11]. However, in both techniques preparation are more difficult than the standard silicone plastination and need a variety of tools and materials such as safety glass plates, gaskets, fold-back clamps, heavy plastic foil, ball bearings, magnet and oven for epoxy and UVA light for polyester curing. Two commonly used techniques are flat chamber and sandwich [2,6,9,12].
There are numerous difficulties in preparing a perfect sheet example by using flat chamber or sandwich method. Accurate placement, equal resin-mix distribution on each side of the section and removing air bubbles are main adversities.
While von Hagens and Steinke et al. previously presented xylene added silicone impregnation, they prepared solid organ plastination models [3,12]. Pendowski et al. performed thin slice silicone plastination. However, they have used standard S10+S3 resin impregnation to prepare 2-3 mm thin pig kidney slices [13]. In this study, we tried to combine these two previous methods and obtain thin silicone sheets with using xylene added silicone resin. We planned to acquire thin, semi-flexible, durable and easy handling cross-sectional samples as educational material for demonstrating the detailed anatomical topography under conditions of a simple plastination laboratory with basic equipment.
Additionally, we discussed a different surface cleaning method from the current literature [2,9]. Surface cleaning is an important step to protect the anatomical structures while cleaning the sawdust on thin slices. Both surfaces of the sections usually covered with sticky sawdust after slicing. This sticky material is filling anatomical spaces, obscuring details, appearing as artifacts on the surfaces and decreasing the visual quality of the finished product.
Materials and Methods
One foot and one hand of two separate cadavers, which were obtained according the relevant law of the country, were used for this study in our anatomy department. Differently from the foot, after washing with 0.9% NaCl solution, the ulnar and radial arteries of the hand injected manually with red colored polyester (Poliya 354 and Polipigment red, Poliya Composite Resins and Polymers, Inc. Turkey). Both specimens were positioned anatomically and precooled at 4°C in refrigerator for one night to prevent from the ice crystal formation during the storage in -80°C for following five days. Frozen specimens are sawed in 2-3 mm thickness (foot on sagittal, hand on frontal plane) using a band saw machine. All slices placed between wire meshes serially. After one month of fixation at room temperature within 10% formalin solution, slices washed under running tap water and scaled to compare with their final weights.
An important factor in terms of visibility of the slice is the surface cleaning by our experiences. At this step, we choose to use a surgical aspirator and a manual water spray. Aspiration was performed by approaching the cross-sectional surface with acute angle while spraying water. Water spraying helps to dislodge the sticky sawdust and provide efficient aspiration. Low levels of vacuum and a lightning magnifier loupe was used for careful cleaning of some areas with thin, delicate and complicated structures.
After complete dehydration at the end of three months in a graded series (95% - 97% - 100%) of cold acetone baths (-20°C), degreasing in the last acetone bath was performed at room temperature within one week as the standard plastination protocol [12,13]. However, in impregnation step we follow up a different protocol: First, we use xylene added (S/X ratio: 1/0.6) silicone reaction mixture (S10+S3, Biodur Products GmbH, Germany); second, the impregnation was performed intermittently at room temperature as described by Zeng et al, [14]. Curing of impregnated slices carried out in a gas-curing chamber in which the specimens exposed to silicone hardener (S6 Hardener, Biodur Products GmbH, Germany) vapor again at room temperature. The added xylene removed out from the slices using vacuum chamber (Kena-Tek Inc. Turkey) after the curing process.
Results
Impregnation has ended in five days at room temperature. The slices were available for routine usage within one week after impregnation. One-week duration needed for curing and xylene removing. The plastinated slices have fine details of many anatomical structures comparable with radiological images; articular relations, cartilaginous surfaces and muscles with their origins and insertions can be determined clearly (Figures 1 and 2). Red polyester filled arteries of the hand are easily traceable (Figure 3). Semi-transparent feature of the sheets provides useful backlit imaging (Figures 2 and 3). Slices are semi-flexible and durable enough for students to use safely in practices (Figure 4).
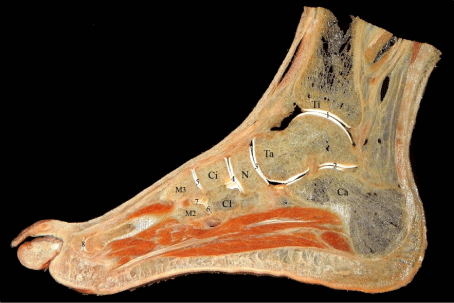
Figure 1: A 2 mm sagittal section of the foot through second and third toes.
Anatomical details are clearly visible. Only bones and articulations were
marked to avoid confusion. Ti: Tibia, Ta: Talus, Ca: Calcaneus, N: Navicular
bone, Ci: Intermediate cuneiform bone, Cl: Lateral cuneiform bone, M2: Base
of second metatarsal bone, M3: Base of third metatarsal bone, 1: Talocrural
joint, 2: Subtalar joint, 3: Talonavicular joint, 4: Cuneonavicular joint, 5-6:
Tarsometatarsal joints, 7: Intermetatarsal joint and 8: Metatarsophalangeal
joint.
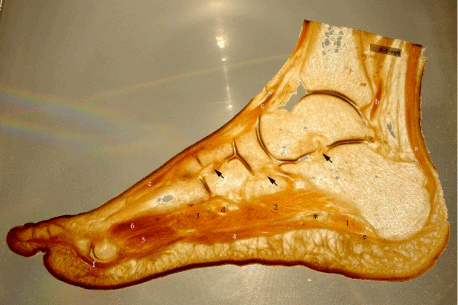
Figure 2: This marked backlit image is another 2 mm section of the foot
through the third toe. Some structures are more distinctive with back light.
a: Calcaneal tendon, b: Tendon of tibialis posterior muscle, c: Tendon of
extensor digitorum longus muscle, d: Tendon of plantaris longus muscle, e:
Plantar aponeurosis, f: Tendon of flexor digitorum longus muscle, asterisk:
Lateral plantar nerve and vessels, 1: Abductor digiti minimi muscle, 2:
Quadratus plantae muscle, 3: Transvers head of adductor hallucis muscle, 4:
Flexor digitorum brevis muscle, 5: Plantar interossei muscles and 6: Dorsal
interossei muscles. Black arrows are pointing the intertarsal ligaments
from right to left: talocalcaneal interosseous, plantar cuboideonavicular and
intercuneiform interosseous ligaments.
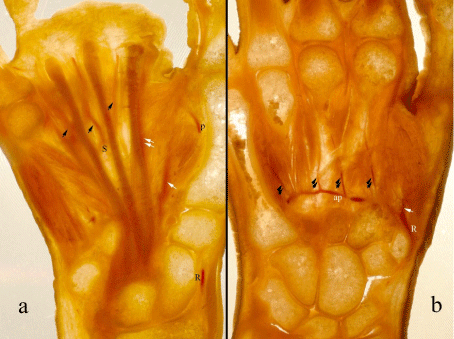
Figure 3: Two different slices of the hand. The superficial (a) and deep
palmar arches (b) and their branches are distinctive in these backlit images.
R: Radial artery, p: Proper palmar digital artery of the thumb, S: Superficial
palmar arch, ap: Deep palmar arch, white arrow: Princeps pollicis artery,
double white arrows: Radialis indicis artery, black arrows: Common
palmar digital arteries, double black arrows: Palmar metacarpal arteries.
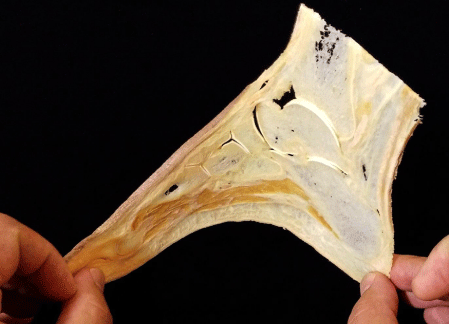
Figure 4: The thin silicone slices are semi-flexible and durable enough for
student practices.
Cleaning surfaces by using surgical aspirator and water spray provide effective remove of the sawdust while protecting anatomical details. There were no artefacts due to residual sawdust on the final products (Figure 5). Using xylene as an additive protects the porous nature of the section surfaces of bones, muscles and joint cavities from filling with silicone.
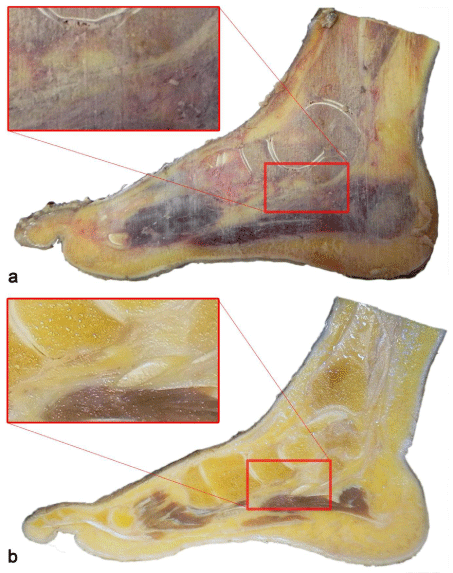
Figure 5: a) The sticky sawdust is coating the slice surface and obscuring
details after saw. b) After surface cleaning with aspiration and water spray:
muscle fibers, joint clearances and spongiform bone sections that previously
obscured with sawdust are visible in detail.
Comparison of weight measurements made before and after plastination showed that, using xylene provides 40% lighter samples than formalin fixed slices.
Discussion
Plastination is a technique of tissue preservation in which water and lipids in biological tissues replaced by curable polymers that are subsequently hardened, resulting in dry, odorless and durable specimens [4,15]. Lightweight plastination is an effective method for obtaining light and elastic materials [3]. Thin epoxy and polyester sheets are breakable and display quality of these materials decreases in time because of surface scratches and UV damage especially for polyester examples [13].
In the process of epoxy and polyester sheet plastination, slices positioned between two plates of glass or stretch film and hollows are filled with polymer. After curing, excessive polymer around the slice necessitates cutting and after correction with wool sander [10]. There is no need for such a process in silicone plastination. It is enough to wipe the sheet’s surfaces once or twice daily, during the curing period. Moreover, sheet silicone plastination can carry out easily in a basic plastination laboratory.There is limited article about thin slice plastination by using standard silicone method in the literature. Pig kidneys are plastinated by using standard S10 plastination technique [13]. Adding xylene into the standard silicone mixture (S10+S3) is a previously defined procedure to get lightweight plastinates but not for sheet plastination [3,12]. We have also repeated this procedure to prepare some solid plastination samples previously and got successfully results [16]. In this study, we combined these two previously defined methods and performed in our laboratory conditions [12,13]. Xylene added S10+S3 mixture and impregnation at room temperature was used to plastinate 2-3 mm thick hand and foot slices.
The arteries of the hand were filled with red colored polyester before sawing. Filling vessels should be done while tissue is fresh and the stuff should be constant in the sliced vessels during fixation and dehydration. We prefer to use red colored polyester (Poliya 354 and Polipigment red, Poliya Composite Resins and Polymers, Inc. Turkey). It is easy to obtain, apply and well harden in fresh tissue.
The missing aspect of this study is the comparison of weights of sheet silicone plastinates with epoxy and polyester sheet samples, as they are not available in our department. For that reason, we choose to compare the slice weights before and after plastination process. At the end, we obtain 40% lighter samples than post-fixation weights with xylene added silicone plastination method. While xylene is available as a domestic product with reasonable prices, silicone is an imported material with much higher costs. Xylene addition reduces the amount of silicone used and shortens the impregnation period by decreasing the viscosity. Moreover, recycled xylene can be use as additive and costs will reduce much more.
Surface cleaning is an important step to reveal the details in sheet plastination. While taking sections the sawdust freeze and adheres on the surfaces. Thickness of the sawdust differs on structural features and is conspicuous on musculoskeletal tissue slices. The sawdust obscures the surface, the anatomical details are untraceable and the material loses its demonstrative features (Figure 5a). Although there are different surface cleaning methods in the literature, like washing with pressured cold water, scrubbing with a brush immediately after sectioning or using CO2 vapor etc [16-19]. Cleaning 2-3 mm thin slices with these methods is not approved and we developed a different technique. After sectioning a fresh frozen material, all slices placed between wire meshes and immersed in to the fixative solution. The surfaces were cleaned with the help of a surgical aspirator and manual water spray after fixation, during the tap water washing period. We preferred to clean the surfaces at this period because the sawdust is loosening and be more appropriate for suctioning. All hallows between tendons, muscle fibers, vessel sections, joint clearances that filled with sawdust were entirely aspirated, and all details revealed (Figure 5b). Using lowest suction speed and acute angled approach to the surface is advising at the beginning to avoid tissue damage. Water spray helps to separate the sawdust from the surface, dislodges tenacious dirt and provides additional wetness for easy suction.
Fascial compartments of foot, muscles, inter tarsal ligaments, retinacular ligaments (skin ligaments) and vessels filled with colored epoxy were demonstrated in previous studies [20-22]. Filling vessels with colored polymer before slicing provides to demonstrate microvasculature even in 3-4 mm thick sheet epoxy plastinates [23,24]. Although not as clear as epoxy and polyester sheets it is possible totrace filled vessels on sheet silicone plastinates. They are semi-transparent and provide the ability to show detailed sectional anatomy (Figures 1-3).
Cross-sectional anatomy is very important in understanding the structural integrity of the human body. It is necessary to diversify the training programs and increase the number and diversity of the training materials. The early encounter of the students with the cross-sectional anatomy will have a significant positive effect to the awareness of how and where to use these anatomical knowledge. When the subject is teaching anatomy with radiological and clinical correlation, silicone sheet plastination can be a preferable solution.
Beside teaching materials, plastinated sheet examples can also use in research studies and provide execution of photogrammetric measurements [25,26].
Conclusion
Today, plastination seems to be the ideal method for preparing and preserving cross-sectional specimens. We believe that silicone plastinated sheets are useful materials for laboratories that do not have adequate equipment or experience to make epoxy and polyester sheet plastinates.
Acknowledgement
No funding was received for this study and the authors declare that they have no conflict of interest.
References
- Von Hagens G. Impregnation of soft biological specimens with thermosetting resins and elastomers. Anat Rec. 1979; 194: 247-255.
- Sora MC, Cook P. Epoxy plastination of biological tissue. J Int Soc Plast. 2007; 22: 31-39.
- Steinke H, Rabib S, Saitoc T, Sawuttic A, Miyakic T, Itohc M, et al. Lightweight plastination. Ann Anat. 2008; 190: 428-431.
- Jones DG. Re-inventing Anatomy: The impact of plastination on how we see the human body. Clin Anat. 2002; 15: 436-440.
- Weber W, Henry RW. Sheet plastination of bodyslices - E12 technique, fillingmethod. J Int Soc Plast. 1993; 7: 16-22.
- Weber W, Weiglein A, Latorre R, Henry RW. Polyester plastination of biological tissue: P35 technique. J Int Soc Plast. 2007; 22: 50-58.
- Steinke H, Pfeiffer S, Spanel-Borowski K. A new plastination technique for head slices containing brain. Ann Anat. 2002; 184: 353-358.
- Sui HJ, Henry RW. Polyester plastination of biological tissue: Hoffen p45 technique. J Int Soc Plast. 2007; 22: 78-81.
- Henry RW, Latorre R. Polyester plastination of biological tissue: P40 technique for brain slices. J Int Soc Plast. 2007; 22: 59-68.
- Chun P, Yu S, Qin H, Sui H. The use of P45 plastination technique to study the distribution of preseptal and preaponeurotic fat tissues in asian eyelids. Cell BiochemBiophys. 2015; 73: 313-321.
- Yuan XY, Yu SB, Li YF, Chi YY, Zheng N, Gao HB, et al. Patterns of attachment of them modular bridge by therectuscapitis posterior minor muscle. AnatSciInt. 2016; 91: 175-179.
- Von Hagens G. Heidelberg plastination folder: collection of technical leaflets of plastination. Biodur products. Heidelberg. 1986; 69126.
- Pendovski L, Petkov V, Popovska-Percinic F, Ilieski V. Silicone plastination procedure for producing thin, semitransparent tissue slices: A study using the pig kidney. J IntSocPlast. 2008; 23: 10-16.
- Zheng TZ, Liu J, Zhu K. Plastination at room temperature. J IntSocPlast. 1998; 13:21-25.
- Von Hagens G, Tiedemann K, Kriz W. The current potential of plastination. AnatEmbryol. 1987; 175: 41-421.
- Bilge O, Celik S, Boduc E. Plastination of old fixed locomotor system specimens and usage in education. Ege J Med. 2014; 53: 84-87.
- Steinke H. Plastinated body slices for verification of magnetic resonance tomography images. AnnAnat. 2001; 183: 275-281.
- Gardetto A, Dabernig J, Rainer C, Piegger J, Piza-Katzer H, Fritsch H. Does a superficial musculoaponeurotic system exist in the face and neck? An anatomical study by the tissue plastination technique. Plastic Reconstr Surg. 2003; 111: 664-672.
- Sora MC, Genser-Strobl B, Radu J, Lozanoff S. Three-dimensional reconstruction of the ankle by means of ultrathin slice plastination. ClinAnat. 2007; 20: 196-200.
- Oppermann J, Franzen J, Spies C, Faymonville C, Knifka J, Stein G, et al. The microvascular anatomy of the talus: a plastination study on the influence of total ankle replacement. Surg Radiol Anat. 2014; 36: 487-494.
- Faymonville C, Andermahr J, Seidel U, Muller LP, Skouras E, Eysel P, et al. Compartments of the foot: topographic anatomy. Surg Radiol Anat. 2012; 34: 929-933.
- Nash LG, Phillips MN, Nicholson H, Barnett R, Zhang M. Skin ligaments: regional distribution and variation in morphology. Clin Anat. 2004; 17: 287- 293.
- Rath B, Notermans HP, Franzen J, Knifka J, Walpert J, Frank D, et al. The micro vascular anatomy of the meta tarsal bones: a plastination study. SurgRadiolAnat. 2009; 31: 271-277.
- Rath B, Notermans HP, Frank D, Walpert J, Deschner J, Luering CM, et al. Arterial anatomy of the hallucal sesamoids. ClinAnat. 2009; 22: 755-760.
- Genser-Strobl B, Sora MC. Potential of P40 plastination for morphometric hip measurements. Surg Radiol Anat. 2005; 27: 147-151.
- Sora MC, Genser-Strobl B. The sectional anatomy of the carpaltunne land its related neurovascular structures studied by using plastination. Eur J Neurol. 2005; 12: 380-384.