
Research Article
Austin J Anat. 2018; 5(3): 1086.
Hypervitaminosis A Causes Reversible Liver Volume Changes Independent of Body Mass in Albino Rats (Rattus Norvegicus)
Munguti JK*, Obimbo MM, Odula PO, Sibuor OO and Cheruiyot IK
Department of Human Anatomy, University of Nairobi, Kenya
*Corresponding author: Munguti JK, Department of Human Anatomy, University of Nairobi, Nairobi, Kenya
Received: October 10, 2018; Accepted: November 05, 2018; Published: November 12, 2018
Abstract
Background: Hypervitaminosis A causes hepatic hyperplasia and fibrosis even in the absence of clinical signs. There is paucity of data on the concomitant changes in absolute and Body Mass-Normalized Liver Volumes (BM-NLV) yet this information might explain the hepatomegaly that precedes other adverse features of vitamin A toxicity.
Objective: To determine the changes in absolute and BM-NLV secondary to acute and persistent hypervitaminosis A.
Methods: After ethical approval, 45 adult albino rats were randomly divided into 3 groups: Group A rats were given 300,000 IU/Kg of vitamin A every alternate day via subcutaneous injection for 4 weeks while Group B rats were given a similar dose but for 8 weeks. Group C rats were the control group. Five rats from group A were euthanized at weeks 2, 4, 6 and 8 while those from group B were euthanized at weeks 6, 8, 10 and 12. The rats were weighed whole while rat liver volumes were estimated using the Scherle method. Means and SD were then determined. The one-way ANOVA was used to compare the volumes for the 3 groups and over time. The Tukey test was the post-hoc test used to detect between which groups the significant difference lay. A p value <0.05 was considered significant at 95% confidence interval.
Results: There was an increase in the absolute liver volume with exposure to high dose vitamin A which continued to rise in spite of stopping the exposure. The BM-NLV on the other hand, showed a marked reversible decline following persistent exposure to high dose vitamin A (p=0.074).
Conclusion: Hypervitaminosis A causes substantial but reversible temporal changes in hepatic volumes.
Keywords: Vitamin A; Hepatotoxicity; Liver volume
Introduction
Vitamin A, a fat-soluble vitamin essential for vision, growth, cellular differentiation and the integrity of the immune system [1,2], has been established to be hepatotoxic when taken in excessive amounts [3-5]. The histological changes seen in hypervitaminosis A induced hepatotoxicity include hyperplasia and vacuolation of hepatic stellate cells, increased collagen fibre deposition with resultant vascular distortion and disruption of hepatic cords [6,7]. These changes have been known to eventually lead to hepatic fibrosis and non-cirrhotic portal hypertension and occur even in the absence of other clinical features [8,9]. There is however insufficient data that illustrate the attendant gross hepatic changes associated with hypervitaminosis A. This study therefore aimed at determining the changes in absolute and Body Mass-Normalized Liver Volumes (BMNLV) secondary to acute and persistent hypervitaminosis A.
Materials and Methods
A total of 45 adult male albino rats (Rattus Norvegicus) were used in this study. Ethical approval to conduct the study was obtained from the Biosafety, Animal Use and Ethics Committee, Faculty of Veterinary Medicine, University of Nairobi. Any rats that had gross malformations or were light for age at the time of commencement of the study were excluded. All animals were handled while fully awake to minimize stress to them and were held by the skin over their back.
Selected rats were randomly divided into 3 groups: A (20 rats), B (20 rats), and C (5 rats). Group A rats were given high dose vitamin A (300,000 IU/Kg) every alternate day via subcutaneous injection for 4 weeks with half of them being followed for a further 4 weeks without the high dose vitamin A treatment. They represented the acutely exposed group. Group B rats were similarly given high dose vitamin A every alternate day via subcutaneous injection for 8 weeks. Half of the members of this group were then followed up for 4 more weeks without being injected with the high dose of vitamin A. This group represented the rats subjected to persistent hypervitaminosis A. Group C were the control group and received subcutaneous injection of sterile normal saline every alternate day over the duration of the study. All injections were done via small hypodermic needles (gauge 25) and only the exact volume of the vitamin solution was injected (150 microliter). To prevent infections, the injection sites were swabbed with surgical spirit. The rats were maintained on a normal diet and fed ad libitum. They were kept in standard and well labelled cages measuring 109cm by 69cm by 77.5cm. Each cage contained 6 animals and was floored with wood shavings which were replaced every two days. Two rats from the control group were used for baseline results.
All rats were weighed at the onset of the study and just before euthanasia was done. Five rats for every week from group A were euthanized at weeks 2, 4, 6 and 8 while those from group B (5 rats for each week) were euthanized at weeks 6, 8, 10 and 12. Euthanasia was performed using gaseous halothane (1-3%) soaked in cotton wool inside an airtight glass jar and was ascertained when the rats showed absent pupillary light reflex and exhibited minimal response to pain. Animals were weighed whole using a digital weighing scale (accuracy of 0.001g). A midline body incision was then made and formal saline infused via the trans-cardiac method. Perfusion was stopped when rigor mortis was complete. The liver was then harvested en masse and stored in formal saline. Its volume was measured thrice using the Scherles’s method of water displacement and an average of the 3 readings recorded (Figure 1). Body Mass-Normalized Liver Volume (BM-NLV) was calculated by dividing the average volume of the liver (cm3) by the respective weight of the animal (g) at euthanasia and multiplying the result by 100. All the remaining carcasses were incinerated at the Department of Veterinary Anatomy and Physiology.

Figure 1: Photograph showing Scherle’s method of liver volume estimation.
Statistical analysis
The obtained data was keyed into the Statistical Package for Social Sciences (SPSS) software (version 24.0, Chicago, Illinois) for coding, tabulation and statistical analysis. Measurements were expressed in cm3 and cm3/g. The data was grouped into three groups as earlier described. Normality of the data was determined using histograms and box plots. Means and the standard deviations were then determined. The one-way ANOVA was used to compare the absolute volume and BM-NLV for the 3 groups and their changes over time. When a significant difference was shown with ANOVA, the Tukey test was used as the post-hoc test to detect between which groups the significant difference lay. A p value <0.05 was considered significant at 95% confidence interval. Results were presented in tables and graphs.
Results
All the animals survived to the end of the experimental period and had appropriate weight gain. In addition, none of the animals had any noticeable gross defects.
Absolute liver volumes
The liver volumes of the control rats remained steady over time and ranged between 11.9 cm3 and 12.7 cm3. Acute exposure to high dose vitamin A, on the other hand, caused a slight elevation of the hepatic volume while persistent injection beyond 4 weeks caused a marginal decline. Stopping the exposure to vitamin A, for both acutely and persistently exposed animals, resulted in continued increase in the liver volumes (Graph 1.1). These changes were, however, not statistically significant (p=0.895).
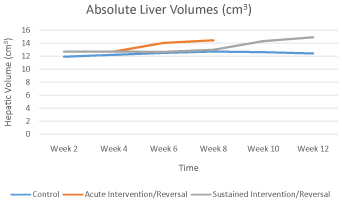
Graph 1.1: Graph showing the trend of absolute liver volume over time.
Body Mass -Normalized Liver Volume (BM-NLV)
The BM-NLV of the control animals remained relatively constant over the entire study period. On the other hand, the BM-NLV for the animals exposed to acute hypervitaminosis A showed a gradual decline up to the 4th week before going up with stopping of injection of vitamin A to peak at week 6. It then returned to the baseline by the 8th week. Prolonged exposure to high dose vitamin A resulted in a more acute decline of the BM-NLV between weeks 6 and 8. However, following cessation of vitamin A administration this decline reversed to near normal levels by week 12 (Graph 1.2). These differences were however, not statistically significant (p>0.05).
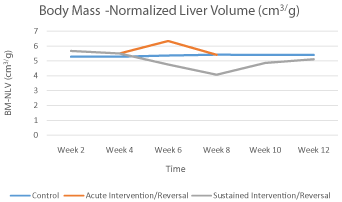
Graph 1.2: Graph showing the trend of the body mass -normalized liver
volume (BM-NLV) over time.
Discussion
In recent times, there has been an increased concern on the risk posed by vitamin A toxicity in the absence of clinical signs. This follows multiple reports documenting several cases of vitamin A induced hepatotoxicity following use of retinoic acid in management of dermatologic conditions and as over the counter prescriptions for nutritional supplements [9]. The negative effects to the liver following enhanced retinoic intake can therefore not be overlooked
[10], like those reported the figures of the absolute liver volumes for the control group in the current study. These near constant figures over time could be attributed to the fact that the animals were recruited into the study as young adults and thus had attained optimal adult liver size. The increase in absolute liver volumes with time in animals exposed to high dose vitamin A is also in keeping with previous reports that have documented hepatomegaly in patients with chronic vitamin A intoxication [11,12]. Such increase has further been known to precede gross morphological changes in animals subjected to toxicological studies [8,13] and is independent of tissue shrinkage secondary to histological processing [14]. This might explain the lack of discernible gross changes seen in the intervention group animals despite the changes in hepatic volumes. Such an increase in the absolute liver volume seems to be an important early sign of hepatic injury secondary to certain hepatotoxins and more so in hypervitaminosis A. Unfortunately, the increase in the liver size secondary to chronic hypervitaminosis A has been known to persist way after cessation of the excess intake of the vitamin [10] which might explain the continued rise of the liver volumes in our study even after cessation of vitamin A injection for both the acute and persistently exposed rats.
The decline in BM-NLV with continued exposure to hypervitaminosis A could be attributed to hepatocyte necrosis secondary to the hepatotoxic effects of the vitamin in the absence of other systemic side effects [9]. With discontinuation of exposure to high dose vitamin A however, the BM-NLV was seen to normalize and this may be associated with the recovery of hepatocyte. Such recovery of the liver following chemotoxin and drug induced injury has been reported [6,7]. However, it has also been noted that even though resolution of chemical induced liver injury may occur with discontinuation of exposure to the injurious agent, full recovery may take much longer and progression of the disease may still occur [15,16]. Close follow up of such patients would therefore be paramount.
Conclusion
Hypervitaminosis A causes significant but reversible temporal changes in hepatic volumes independent of body weight changes. Withdrawal of exposure to vitamin A in cases of suspected toxicity, accompanied by other adjuvant therapies, would therefore be paramount in the management of both acute and chronic hypervitaminosis A.
References
- Genaro P, Martini LA. Vitamin A supplementation and risk of skeletal fracture. Nutr Rev. 2004; 62: 65–67.
- Raoofi A, Asadi F, Mardjanmehr SH, et al. The effects of hypervitaminosis A in sheep following intramuscular administrations of vitamin A. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2010; 48: 193–195.
- Murphy SP. The fitness of FITS. J Am Diet Assoc. 2010; 110: 8-10.
- Radimer K, Bindewald B, Hughes J, et al. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol. 2004; 160, 339–349.
- Yetley EA. Multivitamin and multimineral dietary supplements: definitions, characterization, bioavailability, and drug interactions. Am J Clin Nutr. 2007; 85: 269–276.
- Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007; 131: 1728–1734.
- Pinzani M. Hepatic stellate (ITO) cells: expanding roles for a liver-specific pericyte. J Hepatol. 1995; 22: 700–706.
- Forouhar F, Nadel MS, Gondos B. Hepatic pathology in vitamin A toxicity. Ann Clin Lab Sci. 1984; 14: 304–310.
- Levine PH, Delgado Y, Theise ND, et al. Stellate-cell lipidosis in liver biopsy specimens. Recognition and significance. Am J Clin Pathol. 2003; 119, 254– 258.
- Piao Y, Liu Y, Xie X. Change trends of organ weight background data in sprague dawley rats at different ages. J Toxicol Pathol. 2013; 26: 29–34.
- Geubel AP, De Galocsy C, Alves N, Rahier J, Dive C, et al. Liver damage caused by therapeutic vitamin A administration: estimate of dose-related toxicity in 41 cases. Gastroenterology. 1991; 100: 1701–1709
- Muenter MD, Perry HO, Ludwig J. Chronic vitamin A intoxication in adults: Hepatic, neurologic and dermatologic complications. Am J Med. 1971; 50; 129–136.
- Altunkaynak BZ, Ozbek E. Overweight and structural alterations of the liver in female rats fed a high-fat diet: a stereological and histological study. Turk J Gastroenterol. 2009; 20: 93–103.
- Altunkaynak BZ, Altunkaynak ME. Relationship of body weight and volume of liver. A morphometrical and stereological study. Saudi Med J. 2007; 28, 891–895.
- Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009; 62: 481–492.
- Saad RA, EL-Bab MF, Shalaby AA. Attenuation of acute and chronic liver injury by melatonin in rats. J Taibah Univ Sci. 2013; 7: 88–96.