
Review Article
Austin J Anat. 2021; 8(1): 1098.
Bone Preparation from Embalmed Human Cadavers - A Retrieval and Curation Technique
Nayyar AK* and Ghatak S
Department of Anatomy, All India Institute of Medical Sciences (AIIMS), Jodhpur, Rajasthan, India
*Corresponding author: Ashish Kumar Nayyar, Additional Professor, Department of Anatomy, All India Institute of Medical Sciences, AIIMS, Jodhpur, Rajasthan, India
Received: May 19, 2021; Accepted: May 26, 2021; Published: June 02, 2021
Abstract
Bone preparation involves soft tissue removal, maceration, bleaching and labelling. In the absence of a standardized methodology a large repository of human bones are lost, as most medical colleges do not process bones after the dissection of human cadavers. The present study therefore conducted with the aim of evaluating the least time-consuming and effective method of bone preparation from embalmed and wet specimens.
The method used included a process of maceration, which involves soft tissue removal and then boiling the bones in 60 litres of water for 2 hours. The process of maceration was augmented by adding potassium hydroxide pellets (caustic potash mol. wt. 56.11) after 30 minutes of initiation of boiling; 200-250 gm in the case of male bones and 150 – 200 gm in the case of female bones. After maceration was complete, the bones were bleached by soaking them in 30 – 35 litres of hydrogen peroxide 30% w/v solution (mol. wt. 34.01) for 12-14 hours. The bleached bones were then washed with water and soaked in 30 -35 litres of acetone (extra pure mol. wt. 58.08, boiling point 55.5° – 56°C) for 12 hours to degrease them. The bones dried naturally by spreading them on blotting paper and subsequently painted with a mixture of half a litre of lacquer and half a litre of lacquer thinner.
This study concluded that the preparation of bones using the above method was effective, fast, odourless, and good quality human bones for anatomical study resulted.
Keywords: Soft tissue removal Maceration; Bleaching; Degreasing; Potassium hydroxide
Introduction
Through the ages, anatomy evolved as a foundation of medical education. Teaching anatomy has become a challenging task. Osteology or study of bones are very essential and integral part of anatomy teaching curriculum [1]. Human bones are unsurpassed in the ability to provide three-dimensional instruction in osteology. In addition, bones also assist in understanding the sites of soft tissue insertion and the course of neurovascular bundles in the region. By utilizing a combination of a dried bone with textbooks and atlases as well as anatomy dissection, the important aspect of bone anatomy can be learned most efficiently [2].
Bone preparation essentially involves soft tissue removal, bone bleaching, bone articulation and labelling. The time required for these processes vary depending on the size of the human dead body or the Caracas in case of animals [3]. There are different techniques used in bone preparation, which include insect consumption, cold-or warm -water maceration, which have been used as standard maceration techniques and requires between 2 days and 8 weeks depending on the amount of bacteria present, the size of material being macerated and the temperature of the environment during the maceration. Boiling and subsequent mechanical cleaning of skeletal material is also used [4].
Solutions of organic and inorganic chemicals are also used to remove soft tissue from bones. Inorganic chemicals used are antiformin, ammonium hydroxide, sodium hydroxide and other alkaline solutions. Maceration with organic chemicals can be performed with enzymes such as papain or pepsin or with washing powders containing enzymes [5] as well as burying in soil [1].
Original human bones are not available in the market due to government’s policy of not licensing any vendor to deal in human bones. In the absence of a standardized method of bone retrieval, a large repository of human bones is lost, as most medical colleges do not process the bones after the dissection of human cadavers. Original human bones sold by unauthorized persons are excessively expensive and that too without a valid invoice. Artificial bones made of plaster-of-paris or resins available in the market are inferior in terms of details of the morphological features of the original bones.
The authors, having tried different methods, have been able to standardize their own method and presents the method of retrieving bones from cadavers (or animals) using a combination of various bone cleaning techniques for anatomical studies.
Technical Details and Method of Use
Retrieval of bones from human cadavers includes the following steps:
Step1: Removal of soft tissue: Scrub, pick and (gently) scrape away loosened muscles, ligaments and soft tissues using a scalpel, opening up all the joints of extremities(Limbs) and separating the bones. Articulated hand and foot, vertebral column and pelvis should kept intact at this stage as any attempt to separate them at this stage may cause damage (Figure 1).

Figure 1: Removal of soft tissue.
Step 2: Maceration:
• Soak the bones in tap water for 24 hours for softening. About 30–40 litres of water for bones from one cadaver depending on the size and weight of the body (Figure 2).

Figure 2: Bones soaked in tap water for 24 hours.
• Remove soft tissues, which still left, by gentle scrapping (Figure 3).

Figure 3: Soft tissue removal by gentle scrapping.
• Put the bones in water in an appropriate container ensuring that the bones are completely immersed (about 80 - 100 litres of water) and Boil for 2 hours. Boiling the bones in water must be done carefully and should be checked often. Boiling should be done outdoors when possible. Rigorous boiling can damage skulls. Once the boiling has started, the bones should be gently simmered to prevent damage (Figure 4).

Figure 4: Bones immersed in water and boil for 2 hours.
• Add Potassium Hydroxide (KOH) pellets (Caustic potash Mol. Wt. 56.11) 200–250 gm in case of male bones and 150–200 gm in case of female bones (Figure 5). Add Common Salt half kg (1/2 Kg) to the solution and keep it simmering for 11/2–2 hours. Keep checking the bones. Remove as much flesh as possible. Remove vertebral column and separate the vertebrae. Similarly, the hand and foot can be disarticulated and clean off more of the tissue. The flesh is easy to remove with a knife, scalpel, or pliers.

Figure 5: Treatment with Potassium Hydroxide (KOH) pellets.
• Remove skull, clean off more of the tissue, and repeat this process until the skull is clean. Tying a string to the skull will facilitate picking it out of the hot water. Through the Foramen magnum, the brain must be cut into pieces with scalpel and the brain with duramater should be scooped out. It is important to check the boiling skull frequently. Boiling too long can damage skulls and actually dissolve bone tissue.
• Wash and rinse the bones in tap water at room temperature. Check the bones and remove any soft tissue still adherent to the bones with a scalpel (Figure 6).
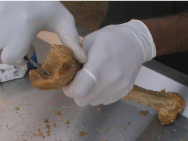
Figure 6: Removal of Soft tissue adherent to bones after boiling.
• Soak the bones in tap water at room temperature for at least 12 hours.
Step 3: Bleaching: Wash and rinse individual bones. Soak all the bones from one cadaver in 30–35 litres of Hydrogen Peroxide (H2O2) 30% w/v solution (M.W. 34.01) ensuring all the bones covered with H2O2. Cover with a lid and keep for 12-14 hours. Keep checking, as over-bleaching will make the bones brittle (Figure 7).
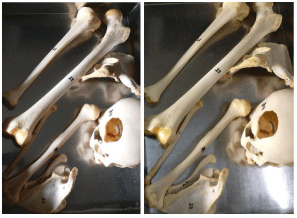
Figure 7: Bones after Bleaching.
Step 4: Degreasing: Wash the bleached bone thoroughly with tap water. Make sure thoroughly rinse the bones in fresh water immediately after subjecting them to the bleach, because the chlorine will continue to degrade the bone surfaces even after they are allowed to dry. Immediate reboiling in fresh or soapy water will also remove the bleach.
Soak them in Acetone extrapure M.W. 58.08 Boiling point (95%) 55.5–56 °C (till all the bones are immersed; about 30 -35 litres) for 12 hours.
Step 5: Drying: Remove the bones from Acetone and wash with tap water. Spread the bones on blotting paper and let the bones dry in normal room temperature (ambient: 36-44 degree Celsius) for 4-5 days.
Step 6: Finishing: Finish the bones, after they are completely dry, by painting with a mixture of half litre lacquer and half litre lacquer thinner (alternately by painting with Johnson Touchwood®). This will prevent erosion at the ends of the bones (Figure 8).
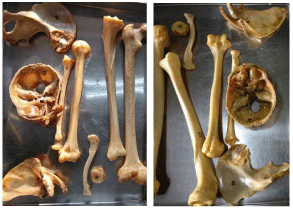
Figure 8: Polished bones.
Disposable latex gloves should be used to protect oneself from both any bacteria and the chemicals and Standard safety precautions to be taken to avoid blood-borne pathogens.
While retrieving bones from specimens preserved in formalin or formaldehyde by boiling as described in this method, facemask must be worn at all times so as not to breathe in the fumes or steam. Exposed skin should be covered while processing, and it has to be ensured that the disposal of the resulting fluid and soft tissues conforms to local regulations.
Salient features:
• The method uses chemicals easily available in any Anatomy department of a medical college or easily available with any vendor dealing in chemicals.
• The chemicals used in the method are inexpensive.
• Soft tissue removal by solutions of chemicals found to be the most effective since it macerates bone in a remarkably fast and odourless way. In addition, has a less degradative effect on bone DNA than the other cleaning processes, which is time consuming especially with human cadaver, and elicits strong odours.
• The method is suitable including Formalin-fixed cadavers as it does not involve using Insects to clean bones, as insects do not eat into formalin fixed tissue. In addition, several thousand are required to produce rapid cleaning and if left with bones for a long period can eat and destroy them.
• The method includes degreasing since grease must be removed because it will smell, seep through the bone, and attract dust and grime.
• The method does not damage the bone and preserves all morphological traits of the bones.
Discussion
Osteology or study of bones are very essential and integral part of anatomy teaching curriculum [1]. Human bones are unsurpassed in the ability to provide three-dimensional instruction in osteology. In addition, bones also assist in understanding the sites of soft tissue insertion and the course of neurovascular bundles in the region. By utilizing a combination of a dried bone with textbooks and atlases as well as anatomy dissection, the important aspect of bone anatomy learned most efficiently [2].
Bone preparation essentially involves soft tissue removal, bone bleaching, bone articulation and labelling. The time required for these processes vary depending on the size of the human dead body or the Caracas in case of animals [3]. There are different techniques used in bone preparation, which include insect consumption, cold-or warm -water maceration, which have been used as standard maceration techniques and requires between 2 days and 8 weeks depending on the amount of bacteria present, the size of material being macerated and the temperature of the environment during the maceration. Boiling and subsequent mechanical cleaning of skeletal material is also used [4].
Solutions of organic and inorganic chemicals also used to remove soft tissue from bones. Inorganic chemicals used are antiformin, ammonium hydroxide, sodium hydroxide and other alkaline solutions. Maceration with organic chemicals can be performed with enzymes such as papain or pepsin or with washing powders containing enzymes [5] as well as burying in soil [1].
Maceration
Bones macerated in solutions of varying pH to observe the effects of varying fresh water pH on bone. Additional simmering in borax helped to break down cartilage and collagen, followed by soaking in xyol to remove residual fats.
Extreme pH levels may be destructive, while more moderate pH levels have lesser but significant effects. The pH 7 and pH 10 solutions have little effect on the bone, but the other solutions affect bone to varying degrees [6].
Maceration using insects: It has been shown that dermestid beetles are useful as a technique to clean bones, especially for the parts of the skeleton, which are difficult to dissect by hand.
An experiment with the harvester termite: Trinervitermes trinervoides (Isoptera: Termitidae) in the Sterkfontein Valley, South Africa to create a reference collection showed that within six months all bones were approximately half covered with a dark surface residue, had an etched surface appearance and recorded boreholes and destruction, particularly of less dense elements and epiphyses. This experiment has demonstrated that T. trinervoides can destroy bone in all stages of preservation, favouring fresh thin cortical and spongy bone with meat and marrow [7].
Use of chemicals: Human skulls are important in osteology because they aid in understanding the sites of soft tissue insertion and their intricate course of neurovascular structures in the skull base. Recent geopolitical developments in Asia have led to extreme difficulty in obtaining human skull specimens. A method for the preparation of dried human skulls from fresh and frozen cadavers using commonly available chemicals has been described [2]. The technique, requiring about 8 weeks’ total time and basic equipment, consists of maceration accelerated by several enzymes followed by defatting, washing and bleaching. The skulls produced are of excellent quality and durability with no preparation artefacts.
An economical source of skulls has now re-established to facilitate learning of the intricate relationships of the skull base. Adult African Giant Pouched 12 rats of both sexes used to determine the effect of three different methods of bone preparation on the bones of the African Giant Rat (Cricetomys gambianus), using chemical preparation with sodium hydroxide. This method found to be the best in terms of time required to complete the procedure, number of bones recovered, colour of the bones and odour of the preparation. However, the chemical method has the disadvantage of dissolving, cracking the bones if the concentration used is high, and prompt attention not given to the preparation [8].
Detergent maceration
Osteological assessment of human remains forms an essential part of forensic work, especially during the examination of extensively decomposed, dismembered, or burnt bodies.
In a study to assess the effectiveness of detergents for the purpose of soft-tissue removal from animal derived specimens, it was shown that such a means is comparable to enzymatic maceration but with fewer health and safety issues and greater advantages regarding transportation and availability of materials when an investigator is in a fieldwork scenario [9].
The forensic biologist called upon to conduct DNA analysis if identification is not possible from visual inspection of skeletal remains. The possibility of downstream DNA testing needs to be considered when skeletal preparation techniques are employed to deflesh human remains, as they have the potential to strongly impact genetic analyses and subsequent identification.
In a study, the effect of 10 maceration methods on gross bone structure and the preservation of DNA in ribs of 12 pigs (Sus scrofa) evaluated. This study shows that traditionally "conservative" maceration techniques are not necessarily the best methods to yield DNA from skeletal tissue [10].
Rennick et al. (2005) employed three cleaning techniques, boiling bone in water, in bleach, and in powdered detergent/sodium carbonate, to test for their effect on nuclear and mtDNA recovery from a variety of human and non-human bones. A statistically significant reduction in DNA yields occurred in non-human bones cleaned with bleach, and DNA degradation was apparent electrophoretically. The human bones also showed much lower yields from bleach cleaning, while the detergent/carbonate method allowed the largest segments of DNA to amplify, indicating it may have a less degradative effect on bone DNA than other cleaning processes [11].
There has been considerable debate among anatomists and anthropologists as to how the remains should be macerated. Many feel that boiling is much too destructive a process. However, we have been experimenting with this method and feel that boiling, when carefully applied, is far superior to no-heat or low- heat methods, beetles, and caustics (bleach, ammonia), and is also quicker. Soft tissues and grease best removed through simmering and boiling. Skin, large bundles of tendon, muscle, and ligaments should be carefully removed with dissecting tools, taking care not to damage the bone surfaces -- the boiling will be doing most of the work [12-14].
Conclusion
We conclude that retrieval of bones using our method is very effective combining various bone-cleaning techniques for anatomical studies. The yield of the bones thus retrieved by this method is of very superior quality in terms of morphological details and can be used for anthropological and morphometric studies.
References
- Modi BS, Puri N, Patnaik VVG. Evaluation of techniques for cleaning embalmed cadavers’s bones. International Journal of Anatomy and Research. 2014; 2: 810-813.
- Ator GA, Andrews JC, Maxwell DS. Preparation of the human skull for skull base anatomic study. Skull base surgery. 1993; 3: 1- 6.
- Boyle C. Maceration and preparation of mammal skeleton for long- term curation. University of Indianapolis: Archaeology and Forensic Laboratory. 2010.
- Eriksen AM, Simonsen KP, Rasmussen AR. Conservation of mitochondrial DNA in fast enzyme-macerated skeletal material, International Journal of Conservation Science. 2013; 4: 127-132.
- Offele D, Harbeck M, Dobberstein RC, von Wurmb-Schwark N, Ritz-Timme S. Soft tissue removal by maceration and feeding of Dermestes sp.: impact on morphological and biomolecular analyses of dental tissues in forensic medicine. International Journal of Legal Medicine. 2007; 121: 341-348.
- Christensen AM, Myers SW. Macroscopic observations of the effects of varying fresh water pH on bone. Journal of forensic sciences. 2011; 56: 475- 479.
- Backwell LR. Parkinson AH. Roberts. Criteria for identifying bone modification by termites in the fossil record. Palaeogeography Palaeoclimatology Palaeoecology. 2012; 337-338: 72-87.
- Onwuama KT, Salami SO, Ali M, Nzalak JO. Effect of different methods of bone preparation on the skeleton of the African giant pouched rat (cricetomys gambianus). International Journal of Morphology. 2012; 30: 425-427.
- Mairs S, Swift B, Rutty GN. Detergent: an alternative approach to traditional bone cleaning methods for forensic practice. The American Journal of Forensic Medicine and Pathology. 2004; 25: 276-284.
- Steadman DW, DiAntonio LL, Wilson JJ, Sheridan KE, Tammariello SP. The effects of chemical and heat maceration techniques on the recovery of nuclear and mitochondrial DNA from bone. Journal of forensic sciences. 2006; 51: 11-17.
- Rennick SL, Fenton TW, Foran DR. The effects of skeletal preparation techniques on DNA from human and non-human bone. Journal of forensic sciences. 2005; 50: 1016-1019.
- Bartels T, Meyer W. A quick and effective method for the maceration of vertebrates. DTW. Deutsche tierärztliche Wochenschrift. 1991; 98: 407-409.
- Simonsen KP, Rasmussen AR, Mathisen P, Petersen H, Borup F. A fast preparation of skeletal materials using enzyme maceration. Journal of Forensic Sciences. 2011; 56: 480-484.
- Ajayi A, Edjomariegwe O, Iselaiye O T. A Review of Bone Preparation Techniques for Anatomical Studies. Malaya Journal of Biosciences. 2016; 3: 76-80.