
Review Article
Austin J Anat. 2021; 8(1): 1099.
In vitro Human Embryo Morphology - An Odissey Outside the Oviduct
Hurtado R1,2, de Lima Bossi R2, Valle M2, Sampaio M2 and Geber S1,2*
¹Department of Obstetrics and Gynaecology of the Medical School, Universidade Federal de Minas Gerais, Brazil
²ORIGEN, Center for Reproductive Medicine, Brazil
*Corresponding author: Selmo Geber, Department of Obstetrics and Gynaecology of the Medical School, Universidade Federal de Minas Gerais, Av Contorno 7747 Belo Horizonte, Minas Gerais, Brazil
Received: May 01, 2021; Accepted: June 09, 2021; Published: June 16, 2021
Abstract
The authors describe the human embryo development in vitro, during the preimplantation stages, i.e., from the zygote until the blastocyst stage. They also describe the methods to select the most suitable embryo for transfer in cycles of In vitro fertilization treatment, performed by infertile patients, in order to increase implantation and pregnancy rates.
Introduction
Couples with the inability to conceive after one year of unprotected intercourse turn to infertility treatment and, in many cases, Assisted Reproductive Technologies (ART)/ In vitro Fertilization (IVF), a technique responsible for the birth of more than 8 million babies worldwide since 1978. Embryo early development and morphology hold important information gathered from basic science that can be extensively applied to ART and, therefore, optimize the outcomes.
The lack of treatment options for improving the quality of sperm and eggs is addressed by increasing gamete quantity. For women, multiple follicular developmet, to increase oocyte number, is induced by gonadotropins in a single menstrual cycle. Moreover, in order to increase pregnancy rates, more than one embryo is transferred for the uterus. Therefore, IVF might increase the rates of multiple pregnancies and premature delivery with consequences on public health [1]. There is no debate that a better embryo selection could downsize the number of transfered embryos per cycle, and therefore decrease the incidence of multiple pregnancies [2].
Even after decades of upcoming technologies for better evaluation of embryos, traditional morphology assessment through a binocular microscope is still the first-line method for embryo selection to transfer in IVF cycles, for none of the more recent technologies have been proven superior [3-5]. On the other hand, morphological assessment does not detect chromosomal abnormalities or defects in critical cellular processes and metabolism that could impact the viability of an embryo. For these purposes, the methods being used to evaluate chromosomal abnormalities of embryos are Pre- Implantation Genetic Testing (PGT) [6] after embryo biopsy or using a non-invasive technology; and for embryonic metabolism, technologies collectively called ‘omics’ which may include genomic, proteomic, transcriptomic and metabolomic profiling of the embryos [7]. Chromosomal analysis has been heavilly criticized since its early years using Fuorescence In-Situ Hybridization (FISH) all the way down to modern PGT-A because it is very well documented that embryos are compartmentalized. This means that the DNA of an abnormal cell within an embryo could be present in one part of the embryo and absent in the others [8]. Also, there is a possibility of an auto correction during development [9].
Prospective randomized trials, which would be the most reliable types of studies for generating evidence-based guidelines, are somewhat difficult to perform considering human embryos. This is the case for reasons highlighted by Matchinger in 2013: a) there is no way to establish a direct correlation between specific embryos transfered and viable ongoing pregnancies observed as result; b) Single-embryo transfers are still not widely used across fertility clinics; c) embryo grading criteria and patient selection may vary significantly from clinic to clinic and from study to study. The result for this situation is that most data concerning embryo assessment derives from retrospective and/or small-sampled studies. In recent years, several international scientific forums have been dedicated to standardize embryo quality assessments in order to promote better embryo selection, producing comparable results for different types of studies and, therefore more reliable guidelines.
Morphological Parameters in Embryo Assessment
Currently, embryo selection is based on embryo morphology and the rate of embryo development in culture. This method is subjective because evaluations are based on the number of blastomeres in the embryo, symmetry of the blastomeres and degree of fragmentation. Also, human embryonic development follows a specific sequence of events where morphological characteristics are defined at determined points in time. In result, embryo evaluation can be carried out either by sequential assessments or a ‘cumulative’ one-time approach [10].
More recently a new emerging thechnology is being tested in order to evaluate embryo developmente and improve selection and implantation. Although the use of time-lapse microscopic photography associated to Artificial Intelingence is increasing and gaining more attention there is no data suggesting an outvome improvement for ART.
Zona Pellucida
Zona pellucia is a non-cellular glycoprotein layer surrounding the plasma membrane of oocytes and embryos, secreted during oogenesis. It has been speculated that both the thickness and the diameter of the Zona Pellucida in hatching embryos could be used as markers for embryo quality. It is known that it is easier for invivo- produced embryos to hatch in comparison to in-vitro-produced embryos. These embryos present their zona pellucida with smaller diameter and thickness. It is thought that this is due to a different molecular composition of the zona as a consequence to the exposure to the oviducts fluids, resulting in a more rigid structure that is more prone to crack [11].
Perivitelline space
Perivitelline space is located between the zona pellucida and the plasma membrane. Aside from some very scarce information concerning size and contents in in-vivo and in-vitro produced embryos, researchers have been paying very little attetion to the periviteline space [12] As pointed out by [13] , this space provides information related to culture conditions and therefore can suggest embryo quality. A reduction in periviteline space could translate as swelling of blastomeres, a marker for poor embryo quality directly related to less cell compaction at the morula stage [14].
Pro-nucleus
The first assessment of an embryo should be at the zygote stage for the appearance of two pronuclei (the first sign of successful fertilization). The Pronuclear scoring systems usually assess the number and relative position of the Nucleolar Precursor Bodies (NPB), pronuclear size and aligment (similar size with central location) and appearance of the cytoplasm [15,16] (Figure 1). These features proved to have a predictive relation to blastocyst formation with increased implantation potential [17,18]. These studies were later challenged by robust data showing lack of consistency for pronuclear scoring systems as valid embryo selection criteria [19] (Table 1).
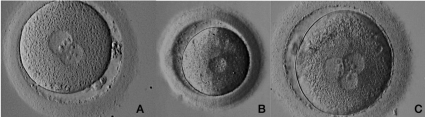
Figure 1: Fertilization and human Pro-nucleus formation.
A: Oocyte with normal fertilization exhibiting two pronuclei, in ooplasm, with nuclear precursor bodies aligned and two polar bodies in perivitelline space; B: Oocyte
with abnormal fertilization, exhibiting one pro-nucleus in ooplasm; C: Oocyte with abnormal fertilization exhibiting three pronuclei in ooplasm.
Grade
Rating
Description
1
Symmetrical
Equivalentto Z1and Z2
2
Non-symmetrical
Other arrangements, including peripherally sited pronuclei
3
Abnormal
Pronuclei with 0 or 1 NPB
Table 1: Fertilization stage grading system (adapted from the Istanbul Consensus, 2011).
Embryo
The embryo is considered after pronuclei singamia and further successive cell divisions. Morphological scoring has been used to predict the highest implantantion potential for embryos since the beginning of IVF [20]. Good quality parameters include the number and symmetry of blastomeres, the absence of multinucleation, early cleavage to the two-cell stage, and a low percentage of cell fragments in embryos. Some other factors found to increase pregnancy and implantation rates include the bastocoelic cavity expansion state and the cohesiveness and number of the inner cell mass and trophectodermal cells [21] (Figure 2).
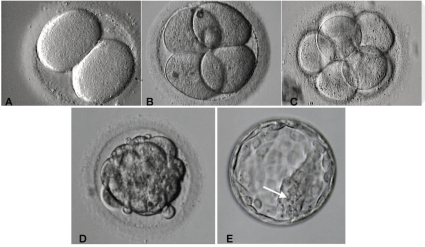
Figure 2: Human embryo development from Day 2 to Day 5.
A,B,C: During embryo development from zygote through cleavage stage, mitotic divisions occur leading a rapid increase in cell number, from day 1 until day 3,
without volume change. Embryo cells in that stage are called blastomeres. (A) the embryo have two blastomeres, (B) four blastomeres, (C) eight blastomeres.
On day four, cell compaction leads to morula formation (D). Day five, two different cell types are present: outside the embryo the trofophectoderm and inside the
embryo the inner cell mass cells (arrow).
Cleavage State
Several international forums have published cleavage-stage scoring systems to determine embryo viability such as the on from the Society for Assisted Reproductive Technology (SART) [22]. Another good example is the Istanbul international consensus panel gathered to come up with an embryo assessment score [23]. The following features are repeatedly present in scoring systems throughout history: the rate of division, symmetry of the blastomeres, multi-nucleation, and the degree of fragmentation. Cytoplasmic colour, the number of cells, and compactation are also frequently observed features.
Colour of the Embryo
Cytoplasmic transluceny of human embryos make it fairly easy to observe important subcellular structures such as the nuclei and the nucleoli, which may be used for pronuclear scoring. In opposition, domestic animals such as pigs, cats and dogs’ embryos have a large number of lipid droplets in the cytoplasm making it quite dark and opaque. Increased lipid content determines lower cryotolerance for embryos which translates as a lower score for embryo quality and transfer selection. A presence of a halo in the blastomeres and concentration of ooplasm in the central part may indicate embryos with a worse prognosis and more susceptible to degeneration. Vacuoles of different sizes and locations may appear in the ooplasm. It is believed that large quantities and large sizes can be harmful to embryo development [24-26].
Cell number
Cell number is still believed to be the single most important indicator for embryo viability [27]. Good quality human embryos evolve from the 1-cell stage to the 16-cell stage along a distinct timeline described by [20] (Figure 3). Embryos that go through cell divisions both ahead or behind the expected time frames are associated with compromised quality [26,28,29], although it has been shown that rapidly cleaving embryos are superior to the late-cleaving, in terms of morula and blastocyst formation [14]. It is also known now that embryos who start the first division between 24-27h are more likely to turn into ongoing pregnancies [30]. Several authors have correlated the number of cells (6 to 8) on day-3 embryos to higher blastocyst formation [31], as well as higher implantation rates [32].
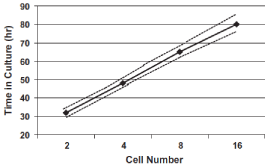
Figure 3: Expected cell development for human embryos; dashed lines
indicate 95% confidence limits (Edwards et al., 1981).
Fragmentation
The presence of fragmentation (anuclear membrane-bound extracellular cytoplasmic structure) has been related to abnormalities in cell metabolism that may result in apoptosis [33,34], anomalies in chromosomal segregation [35,36], and/or abnormalities of the oocyte membrane [37]. Moreover, the presence of fragments can prevent cell-cell interactions, making it difficult to compact, develop blastocele and differentiate in trophectoderm and Inner Cell Mass. There is also a possibility of releasing toxic substances that would compromise embryo development. The simplest scoring system for fragmentation indicates the percentage of the volume of the embryo occupied by fragments (e.g. score 0 = 0%; score 1 = <10%; score 2 = 10–25%; score 3 = >25%) which is negatively correlated with embryo developmental potential and implantation rate (Figure 4). When the embryo starts cleavage, the rate of division, symmetry of the blastomeres, multinucleation and the degree of fragmentation (Table 2) are assessed by the morphological criteria and scored.
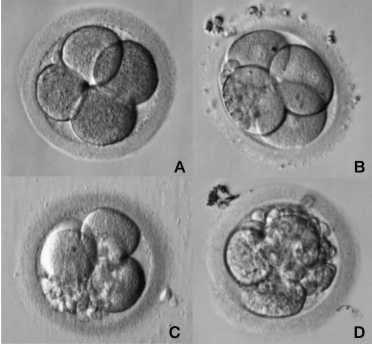
Figure 4: Different degrees of fragmentation in a cleavage stage human
embryo.
During embryo mitotic cell divisions fragmentation may occur varying in
position, degree and size among embryo blastomeres. A: Embryo with no
fragmentation. B: Embryo with less than 10% of fragmentation; C: Embryo with
20-25% of fragmentation; D: Embryo with more than 30% of fragmentation.
Grade
Rating
Description
1
Good
<10% fragmentation, stage specific cell size, no multi-nucleation
2
Fair
Up to 25% fragmentation, stage specfic cellsize for majority of cells, no evidence of multi-nucleation
3
Poor
Severe fragmentation (>25%), cell size not stage specfic, evidence of multi-nucleation
Table 2: Cleavage stage grading system (adapted from the Istanbul Consensus, 2011).
Simmetry
Size and shape of the blastomeres during cleavage may be asymmetrical. This asynchrony in cell division may result from an uneven distribution of organelles, protein and RNA, between sister cells [38]. Embryos with significant asymmetry have been shown to have lower implantation rates [28,39] but not as clearly as compared with the number of cells or fragmentation (Figure 5).
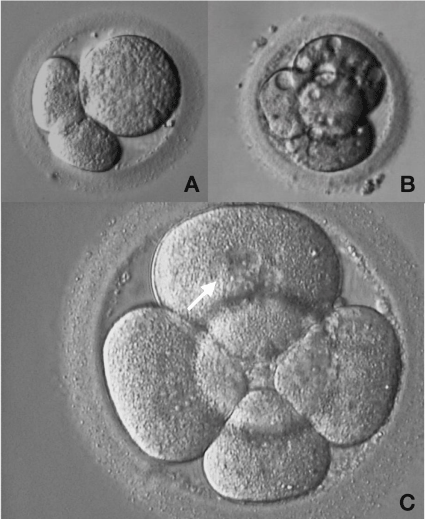
Figure 5: Abnormal human embryo development.
A: Three-cell embryo exhibiting an irregularity in cells size B: Four-cell
embryo with vacuoles inside the blastomeres; C: Five-cell embryo exhibiting
blastomeres with multi nucleation (arrow).
Multinucleation
It has been shown that embryos with more than one nucleus in each blastomere are associated with development arrestment, lower implantantion and lower pregnancy rates due to an increased incidence of chromosomal abnormalities [40-42]. In some cases, however, it might be a transient cell stage self-corrected by the embryo during next cell divisions (Figure 5).
Compaction
Compaction is accompanied by polarization of the embryo's cells. Nearby cells exchange gap junctions for tight junctions and begin to cluster as a result of activating the embryonic genome. A compaction on day 3 of development, in the 8-cell embryo, appears to be indicative of a good prognosis. A loss of maximal compaction, usually detected as an increase of up to 10% in the cell mass area, together with a shorter time period for blastulation are features closely related to aberrant allocation of cells to the inner cell mass and trophectoderm. This phenomenon is secondary to decreased expression of transcripts which are involved in the construction of tight junctions. Moreover, lack of compaction compromises cryosurvival of the subsequently formed blastocysts [43-48].
Blastocysts
Blastocyst stage embryos have two cell types, the Inne Cell Mass cells and the Trophectoderm, in addition to having a cavity called blastocoele. The cells of the ICM form the three embryonic leaflets endoderm, mesoderm and ectoderm and the cells of the trophectoderm will originate the extra-amniotic membranes and the placenta. As the blastocele fills with liquid, the blastocyst increases in size and at its maximum expansion it manages to leave the pellucid zone. The increase in the blastocele occurs due to the active increase in the concentration of salts within the embryo, which cause the liquid to penetrate into the cavity by osmosis. Blastocysts are classified according to their degree of expansion, quality of the trophoblast and quality of the MCI (Figure 6).
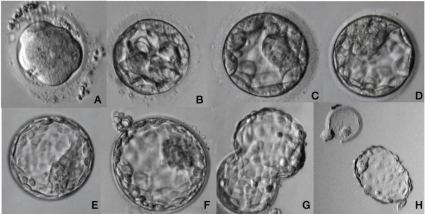
Figure 6: Human blastocyst development.
Embryo compaction initiates on late Day 3 until Day 4 and culminates in morula formation (A). On day 5, cell differentiation occurs and an initial cavity – blastocoele
- is observed (B). Blastocoele is fulfilled with water by osmosis meanwhile trophectoderm and inner cell mass are growing fast (C, D, E). When blastocyst is fully
expanded some cells start the hatching process, to escape from zona pellucida (F, G). Blastocyst is ready for implantation when he was completely hatched from
zona pellucida (H).
Morphological evaluation of blastocysts was first studied by Dokras in 1991 and 1993 but this initial assessment had no proven clinical utility. Later, a more practical score system was proposed by Gardner and Schoolcraft in 1999 and 2000 taking into consideration a specific morphological feature: the extent to which the volume of the embryo is occupied by the blastocoele (Table 3). It was noticed that the number and organization of cells in the inner cell mass and trophectoderm were also important.
Blastocyst stage
Grade
Characteristics
Early blastocyst
1
The blastocoele is less than half the volume of the embryo
Blastocyst
2
The blastocoele is greater than or equal to half of the volume of the embryo
Full blastocyst
3
The blastocoele completely fills the embryo
Expanded Blastocyst
4
The blastocoele volume is larger than that of the early embryo and the zona pellucida is thinning
Hatching blastocyst
5
The trophectoderm has started to herniate through zona pellucida
Hatched blastocyst
6
The blastocyst has completely escaped from the zona pellucida
Inner cell mass
A
Tightly packed, many cells
B
Loosely grouped, several cells
C
Very few cells
Trophectoderm
A
Many cells forming a tightly knit epithelium
B
Few cells
C
Very few cells forming a loose epithelium
Table 3: Blastocyst grading system (Adapted from: Gardner and Schoolcraft, 1999).
More recently both the Society for Assisted Reproductive Technology (SART) [22] and the Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011 developed standardized grading systems which included a more detailed scheme (Table 4).
Grade
Rating
Description
Stage of Development
1
Early Blastocyst
2
Expanded
3
Hatched/Hatching
Inner Cell Mass
1
Good
Prominent, easily discernible, with many cells that are compacted and tightly adhered
2
Fair
Easily discernible, with many cells loosely grouped together
3
Poor
Difficult to discern with few cells
Trophectoderm
1
Good
Many cell forming a cohesive epithelium
2
Fair
Few cells forming a loose epithelium
3
Poor
Very few cells
Table 4: Blastocyst stage scoring system (adapted from the Istanbul Consensus, 2011).
References
- Simlara SL, Rottemberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400135 treatment cycles. Hum Reprod. 2011; 26: 1768-1774.
- Sanchez, et al. Will noninvasive methods surpass invasive for assessing gametes and embryos? Fertility and Sterility. 2017; 108: 730-37.
- Van Soom, et al. Assessment of mammalian embryo quality: what can we learn from embryo morphology? RBMOnline. 2003; 7: 664-670.
- Montag M, Liebenthron J, Ko¨ster M. Which morphological scoring system is relevant in human embryo development? Placenta. 2011; 32: 252–256.
- Rocha, et al. Methods for assessing the quality of mammalian embryos: How far we are from the gold standard? JBRA Assisted Reproduction. 2016; 20: 150-158.
- Palini S, Galluzzi L, De Stefani S, Bianchi M, Wells D, et al. Genomic DNA in human blastocoele fuid. Reprod Biomed Online. 2013; 26: 603-610.
- Seli E, Robert C, Sirard MA. OMICS in assisted reproduction: possibilities and pitfalls. Molecular Human Reproduction. 2010; 16: 513-530.
- Northrop LE, Treff NR, Levy B, Scott RT. SNP microarray-based 24 chromosome aneuploidy screening demonstrates that cleavage-stage FISH poorly predicts aneuploidy in embryos that develop to morphologically normal blastocysts. Mol Hum Reprod. 2010; 16: 590-600.
- Bolton H, Graham SJL, der Aa NV, Kumar P, Theunis K, Gallardo EF, et al. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun. 2016; 7: 11165.
- Geber S, Sales L, Sampaio M. Laboratory techniques for human embryos. Reprod Biomed Online. 2002 5: 211-218.
- Holm P, Booth PJ, Callesen H. Kinetics of early in-vitro development of bovine in-vivo and in-vitro derived zygotes produced and/or cultured chemically defined or serum-containing media. Reproduction. 2002; 123: 553–565.
- Rizos D, Fair T, Papadopouloz S, et al. Developmental qualitative and ultrastructural differences between ovine and bovine embryos produced in vivo or in vitro. Molecular. Betteridge KJ. Phylogeny, ontogeny and embryo transfer. Theriogenology. 1995; 44: 1061–1098.
- Van Soom A, de Kruif A. A comparative study of in vivo and in vitro derived bovine embryos. Proceedings of the 12th International Congress on Animal Reproduction, The Hague, The Netherlands. 1992; 23–27: 1365–1367.
- Scott LA, Smith S. The successful use of pronuclear embryo transfers the day following oocyte retrieval. Hum. Reprod. 1998; 13: 1003–1013.
- Tesarik J, Greco E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum. Reprod. 1999; 14: 1318–1323.
- Zollner U, Zollner KP, Hartl G, Dietl J, Steck T. The use of a detailed zygote score after IVF/ICSI to obtain good quality blastocysts: the German experience. Hum. Reprod. 2002; 17: 1327–1333.
- Nagy ZP, Dozortsev D, Diamond M, Rienzi L, Ubaldi F, Abdelmassih R, et al. Pronuclear morphology evaluation with subsequent evaluation of embryo morphology significantly increases implantation rates. Fertil. Steril. 2003; 80: 67–74.
- Skiadas CC, Racowsky C. Development rate, cumulative scoring and embryonic viability. In: Elder, K., Cohen, J. (Eds.), Human Preimplantation Embryo Selection. Taylor and Francis, Colchester, UK. 2007: 101–121.
- Edwards, et al. The growth of human preimplantation of embryos. Am J Obstet Gynecol. 1981; 141: 408–416.
- Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000; 73: 1155-1158.
- Racowsky C, Stern JE, Gibbons WE, Behr B, Pomeroy KO, Biggers JD. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil. Steril. 2011; 95: 1985–1989.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod. Biomed. 2011; 22: 632–646.
- Lundqvist M, Johansson U, Lundkvist Ö, et al. Does pronuclear morphology and/or early cleavage rate predict embryo implantation potential? Reproductive BioMedicine. 2000; 2: 12–16.
- Montag M, van der Ven H. Evaluation of pronuclear morphology as the only selection criterion for further embryo culture and transfer: results of a prospective multicentre study. Human Reproduction. 2001; 16: 2384–2389.
- Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture invitro. Hum Reprod. 2000; 15: 2634–2643.
- Machtinger R, Racowsky C. Morphological systems of human embryo assessment and clinical evidence. RBM Online 2013; 26: 210– 221.
- Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzman J, et al. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995; 10: 2427–2431.
- Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod 1997: 12: 1545–1549.
- Shoukir Y, Campana A, Farley T, Sakkas D. Early cleavage of in-vitro fertilized embryos to the 2-cell stage: a novel indicator of embryo quality and viability. Hum Reprod. 1997; 12: 1531–1536.
- Jones G, Trounson A, Lolatgis N, Wood C. Factors affecting the success of human blastocyst development and pregnancy following in vitro fertilization and embryo transfer. Fertil Steril. 1998; 70: 1022–1029.
- Racowsky C, Combelles C, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod Biomed Online. 2003; 6: 323–331.
- Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod. 1996; 2: 93–98.
- Perez GI, Tao XJ, Tilly JL. Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol Hum Reprod. 1999; 5: 414–420.
- Pellestor F, Girardet A, Andre´o B, Arnal F, Humeau C. Relationship between morphology and chromosomal constitution in human preimplantation embryo. Mol Reprod Dev. 1994; 39: 141–146.
- Munne´ S, Alikani M, Cohen J. Monospermic polyploidy and atypical embryo morphology. Hum Reprod. 1994; 9: 506–510.
- Fujimoto VY, Browne RW, Bloom MS, Sakkas D, Alikani M. Pathogenesis, developmental consequences, and clinical correlations of human embryo fragmentation. Fertil Steril. 2011; 15: 1197–1204.
- Rienzi L. Significance of morphological attributes of the early embryo. Reprod BioMed Online. 2005; 10: 669–681.
- Hardarson T, Hanson C, Sjogren A, Lundin K. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod. 2001; 16: 313–318.
- Saldeen P, Sundstrom P. Nuclear status of four-cell preembryos predicts implantation potential in in vitro fertilization treatment cycles. Fertil Steril. 2005; 84: 584–589.
- Kligman, I, Benadiva C, Alikani M, Munne’ S. The presence of multinucleated blastomeres in human embryos is correlated with chromosomal abnormalities. Hum Reprod. 1996; 11: 1492–1498.
- Ambroggio J, Gindoff PR, Dayal MB, Khaldi R, Peak D, Frankfurter D, et al. Multinucleation of a sibling blastomere on day 2 suggests unsuitability for embryo transfer in IVF-preimplantation genetic screening cycles. Fertil Steril. 2011; 96: 856–859.
- Miller DJ, Eckert JJ, Lazarri G, et al. Tight junction mRNA expression levels in bovine embryos are dependent upon the ability to compact and in-vitro culture methods. Biology of Reproduction. 2003; 68: 1394–1402.
- Alikani M, Sadowy S, Cohen J. Human embryo morphology and developmental capacity. In: Van Soom A, Boerjan ML (eds) Assessment of Mammalian Embryo Quality: Invasive and Non-invasive Techniques. Kluwer’s Academic Publishers, Dordrecht, The Netherlands. 2002; 1–31.
- Brison DR, Houghton FD, Falconer D, Roberts SA, Hawkhead J, Humpherson PG, et al. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum Reprod. 2004; 19: 2319-2324.
- National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. Page last reviewed. 2021.
- Noci I, Fuzzi B, Rizzo R, Melchiorri L, Criscuoli L, Dabizzi S, et al. Embryonic soluble HLA-G as a marker of developmental potential in embryos. Hum Reprod. 2005; 20: 138-146.
- Nygren KG, Sullivan E, Zegers-Hochschild F, Mansour R, et al. International committee for monitoring assisted reproductive technology (IC MART) world report: assisted reproductive technology 2003. Fertil Steril. 2011; 95: 2209- 2222.
- Reproduction and Development. 2002; 62: 320–327.