
Review Article
Austin J Anat. 2022; 9(1): 1106.
Progress of CT-Based Radiomics in Lung Diseases and Application Prospects in Children
Zhang Y1#, Zhao J1#, Chang Y1 and Zhao C2*
1Department of Pediatrics, the Jinan Children's Hospital Affiliated to Shandong University, China
2Department of Pediatrics, Qilu Hospital of Shandong University, China
#These authors contributed equally work
*Corresponding author: Cuifen Zhao, Department of Pediatrics, Qilu Hospital of Shandong University, China
Received: July 19, 2022; Accepted: August 20, 2022; Published: August 27, 2022
Abstract
Imaging examination, as a powerful clinical technique, is essential for the early diagnosis, disease evaluation and prognostic assessment of lung diseases. As a research hotspot at present, radiomics has become an important part of precision medicine and can provide valuable diagnostic, prognostic or predictive information through omics analyses. By the integration of data analysis, new diagnostic and prognostic biomarkers can be identified in biomedical images. This review provides the latest advances in the application of radiomics in the diagnosis and treatment of lung diseases in adults and children and explores its opportunities and challenges in the precision medicine of lung diseases in children.
Keywords: Lung Diseases; Radiomics; Children
Introduction
In February 2015, the National Science and Technology Ministry and Health and Family Planning Commission established China's precision medical strategy expert group and formulated the "precision medical" strategic plan. It has been included in the major science and technology projects of the "13th Five-Year Plan" in our country. In 2016, the Chinese Medical Association took "Precision medicine of respiratory diseases" as the theme to discuss the relevant issues of the accurate diagnosis and treatment of respiratory diseases for the first time. In the era of precision medicine, the development of radiomics brings challenges and opportunities for the accurate diagnosis and treatment of lung diseases in children.
Radiomics is a new interdisciplinary field and a fusion product of large data technology and medical images to aid diagnosis. The concept of radiomics was first proposed by the Dutch scholar Kumar in 2012 [1]. The emphasis of radiomics is the extraction of large amounts of image data information from images (Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET), etc.) by high-throughput approaches. Radiomics can gather information from different sources for deeper excavation, prediction and analysis to quantify disease characteristics, establish disease models, and identify new diagnostic and prognostic biomarkers to assist physicians in making the most accurate diagnosis [2,3]. Recently, it has evolved into a method that involves the use of imaging, gene, and clinical information for auxiliary diagnosis, analysis and prediction.
CT has significant advantages in the study of lung diseases due to its high resolution for lung tissue, which lays a dependable foundation for providing structural and functional data for further study. Radiomics converts medical images into high-dimensional data, which are then analysed for decision support [2,4]. Radiomics also has great potential for the accurate diagnosis and treatment of lung diseases. Accurate diagnosis is a difficult problem in paediatric imaging. Therefore, our review provides the latest advancements in radiomics application for the diagnosis and treatment of lung diseases in adults and children and explores the opportunities and challenges in precision medicine for lung diseases in children.
The Application of Radiomics in Lung Tumours
Radiomics has shown independent prognosis and prediction capacity in many tumours, especially lung tumours [2] (Figure 1). In the early stages of lung tumours, Solitary Pulmonary Nodules (SPNs) are common findings in thoracic imaging in most patients. In pulmonary nodular diseases, chest CT technology not only provides three-dimensional volume data but also shows various morphological and texture features. Therefore, it can be used to distinguish infection from lung cancer and differentiate benign from malignant SPNs [5,6]. In addition, chest CT technology plays a very important role in increasing the accuracy of diagnosis, reducing the application rate of invasive examination, and assessing the risk of lung cancer progression [7-13]. Moreover, Xue et al. found that different densities of nodules combined with a radiologic model of fractal dimension can distinguish non-invasive adenocarcinoma from invasive adenocarcinoma [14]. Interestingly, Yoon et al. found that lung cancer with ALK/ROS1/RET fusion gene positivity has certain clinical and imaging characteristics [15]. Therefore, it is helpful to identify lung adenocarcinoma with different fusion genes. The features of quantitative images collected by pre-treatment CT are especially efficacious in predicting the shrinkage of tumours and providing additional information about the risk level of patients, treatment, and prognosis for clinical decision-making [16]. In addition, imaging has been used to predict lung cancer gene phenotypes and mutations [17].
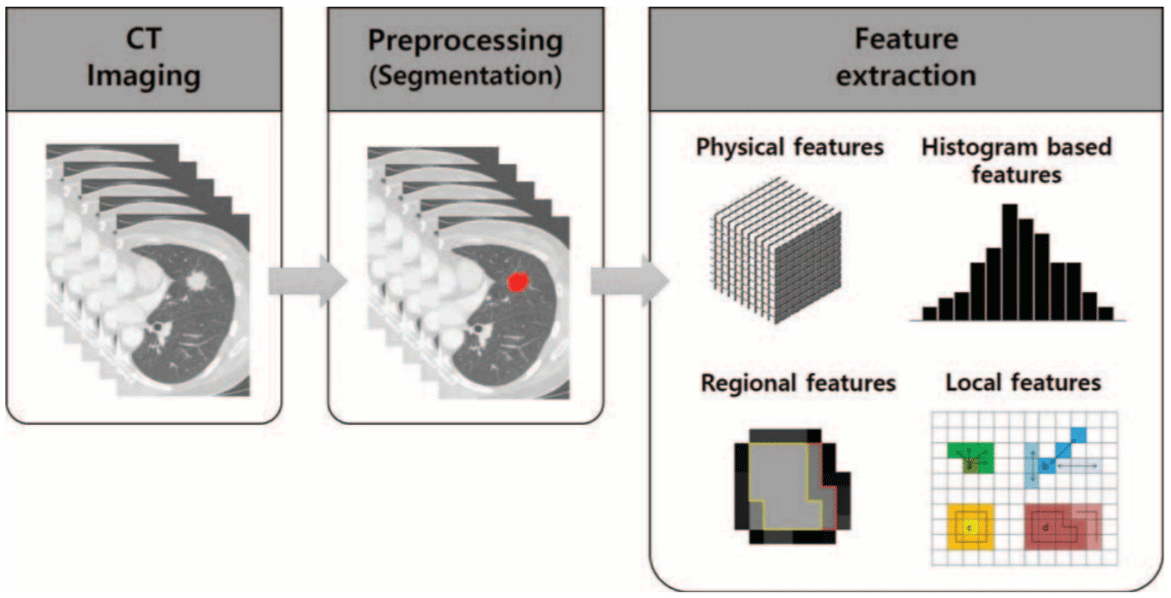
Figure 1: Radiomics Approach in Lung Tumor. Hyun Jung Yoon, et al. Medicine (Baltimore), 2015.
The Application of Radiomics in Pneumonia
Because of the differences in pathogenic factors and reactivity of organisms, as well as the characteristics of lesions and the scope of involvement, infectious pneumonia has become the most common form of pulmonary inflammatory disease. Most lung injuries due to infection are reversible. However, some lung injuries caused by special pathogens or aetiologies, for example, pneumonia caused by Mycoplasma pneumoniae, adenovirus or coronavirus, or aspiration pneumonia, can cause chronic lung injury or complicated chronic pulmonary diseases, such as pulmonary fibrosis and bronchiolitis obliterans, especially in children. Radiomics can extract massive information to indicate the internal characteristics of lesions [18]. By using computer-assisted texture-based image analysis, quantitative assessment of high-resolution CT and disease assessment can be realized adequately in general interstitial pneumonia [19]. The characteristics of grey first order statistics, run length parameters, and co-occurrence matrix features are extracted. The image area of the lesion is also divided into normal and multi parameter areas with discriminant analysis methods. The accuracy rate of classification for the image area of ground-glass opacity lesions was 70.7%.
The main symptom of paraquat poisoning is lung injury. Early manifestations are inflammatory reactions, gradual ground-glass ground glass density shadows, and interstitial injuries, which are difficult to distinguish from pneumonia. Compared with simple imaging, the use of clinical data and establishment of a comprehensive radiomic model could well predict the damage scope and pathological changes in lung lesions and can also reflect the comprehensive and real progress of the patient's disease [20]. In the latest research progress of Artificial Intelligence (AI) technology, the novel coronavirus pneumonia diagnostic system can not only achieve pneumonia diagnosis but also further differentiate the new coronavirus pneumonia from other types of pneumonia (viral pneumonia, bacterial pneumonia, etc.), which takes only a dozen seconds. The coincidence rate with the positive result of nucleic acid detection of the new coronavirus was more than 95.5% [21] (Figure 2). In addition, different image group labels can extract different image group characteristics, which can be used to identify chronic lung injury and pneumonia.
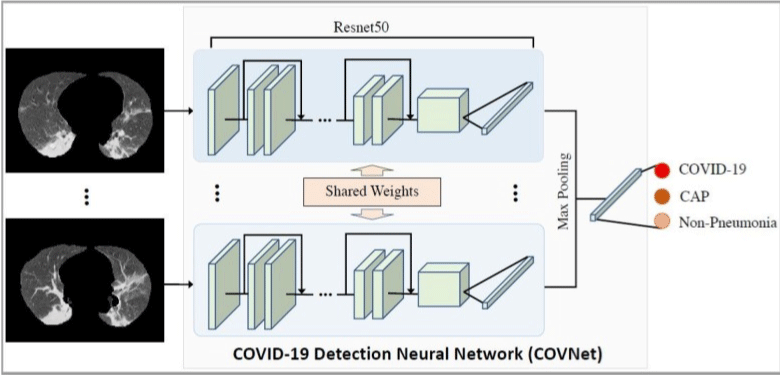
Figure 2: The application of radiomics in pneumonia. Lin Li, Lixin Qin, Zeguo Xu, et al. Radiology Actions Search in PubMed Search in NLM Catalog Add to Search,
2020.
The CT manifestations of invasive mucinous adenocarcinoma are diverse and are difficult to distinguish from those of bacterial pneumonia [22]. Hyun Jung Koo et al. processed the edge and texture of the lesion using a computer [23]. Their research indicates that the efficiency of differentiation between invasive mucinous adenocarcinoma and bacterial pneumonia can be improved by the edge analysis of drawing attenuation changes, with a sensitivity of 86.7% and a specificity of 67.5%. Moreover, there are potential advantages in predicting radiation pneumonitis in lung cancer patients by using changes in CT image parameters [24]. Therefore, clinical diagnosis based on chest CT radiomics can effectively identify cancer and lung infection, which is beneficial to individualize treatment for patients.
The Application of Radiomics in Chronic Lung Diseases
The incidence rate of chronic diseases of the lung is increasing year by year due to the aggravation of air pollution. High-Resolution CT (HRCT) has shown advantages in detecting and quantifying various chronic lung diseases. To achieve accurate diagnosis and treatment, it is necessary to break through the traditional medical image model based on morphology and semi quantitative analysis. Exploring the progress of CT-based radiomics opens up a new way to achieve the accurate diagnosis and treatment of chronic lung diseases. Recently, real-time processing of CT scans has been applied to analyse the characterization and quantification of lung parenchyma morphology by a computational platform named CALIPER (Computer Aided Lung Informatics for Pathology Evaluation and Rating) [25,26] (Figure 3).
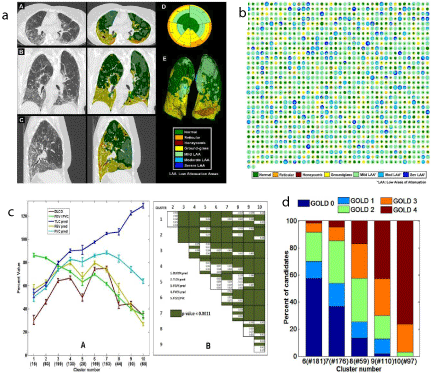
Figure 3: The application of radiomics in pneumonia and chronic lung diseases. a. Computer Aided Lung Informatics for Pathology Evaluation and Rating
(CALIPER). b.The classified parenchymal patterns are represented in the COPD cases and fibrotic cases. c. Image Texture Analysis and mean physiologic
measures. d. The distribution of the old GOLD category and new GOLD category in the correspondence of fibrotic and obstructive clusters with established indices
and disease classification. Sushravya Raghunath, Srinivasan Rajagopalan, Ronald A Karwoski, et al. PLoS One. 2014.
The Application of Radiomics in COPD
Chronic Obstructive Pulmonary Disease (COPD), a common disease in respiratory departments, is associated with persistent airflow restriction and requires careful management for prevention and treatment. COPD is caused by small airway disease (obstructive bronchitis) and lung parenchymal destruction (emphysema). However, the proportions of lesions in COPD patients were different [27]. Currently, the features of COPD on chest CT have been described and measured as direct signs of the destruction of lung parenchyma and emphysema, including bronchial wall thickening and gas trapping as direct and indirect signs of obstructive airway disease [28-30]. Interestingly, some studies on COPD and asthma in adults have shown that quantitative CT measurements are significantly correlated with Pulmonary Function Test (PFT) results [31-34].
With the development of quantified CT metrics and radiomics, the mathematical model of airway function based on standard vital capacity can be used to analyse the existence and severity of emphysema in patients with COPD [35]. The volume of pulmonary emphysema and air-trapping retention in patients with COPD can also be relatively quantified and positioned by using the parameters of the low attenuation area in CT scans, which are predetermined by the X-ray attenuation threshold in the inhalation and exhalation phases [28-36]. The visual manifestations and severity of emphysema, which may reflect the severity of small airway disease, are significantly correlated with the risk of death [37-40]. Meanwhile, quantitative and qualitative studies of lung CT can also distinguish the subtypes of emphysema, namely, the lobular central type, lobular whole type, and lobular paralobular type [41]. Interestingly, according to the study of Occhipinti M et al, a probabilistic model based on Body Mass Index (BMI), with Forced Expiratory Volume in 1 second (FEV1), forced expiratory volume (in one second)/vital capacity (FEV1/VC) and diffusion capacity for carbon monoxide (DLCO) as the percentage of predicted, has been demonstrated to be effective in estimating emphysema in quantitative CT. In addition, emphysema can be estimated by the standard CT metrics of parenchymal destruction by using % LAA-950insp (the relative volume of the attenuation region is less than -950 HU at inspiration in the lung) and the percentage of persistent low-density area [42]. Moreover, Charbonnier JP et al. found that using a quantitative CT model can predict the incidence of COPD in smokers with independent predictors of the incidence rate of COPD, such as LAA%-950, LAA%-856 (the relative volume of the attenuation region is less than –856 HU at inspiration in the lung), and Pi10 (wall thickness in the airway with a diameter of 10 mm). The study of Charbonnier JP et al. also indicated that the parametric spectrum of the lung (PRM) is a tool for the classification of the quantitative density of COPD [43]. Hence, we could distinguish the degree of change in emphysema or airway wall abnormality and the phenotypes of the disease by visual evaluation and quantification of CT, and it can provide a basis for the personalized treatment of COPD by using the measurement of clinical and physiological functions combined.
COPDGene® 2019 recommends redefining the diagnosis of COPD through a comprehensive approach of environmental exposure, clinical symptoms, CT imaging, and spirometry standards. These expanded criteria provide the potential to stimulate current and future interventions that can slow disease progression in patients before irreversible changes in lung structure occur [44]. Cho MH et al. showed the relationship between gene and image subtypes in 12031 patients with COPD, which opened a new field for the differential genetics of COPD phenotypes [45].
The Application of Radiomics in Interstitial Lung Diseases
Interstitial Lung Disease (ILD) is a group of diseases with a diffuse distribution in two lungs. ILD can be divided into seven types according to the causes of the disease, in which Idiopathic Pulmonary Fibrosis (IPF) is the most common, accounting for 47%- 71% of ILD cases [46]. Quantitative analysis of chest CT images can identify and quantify pulmonary fibrosis [26,47]. The main imaging features of ILD on CT images are ground-glass shadows, honeycomb shadows, reticular shadows, consolidation shadows, etc. Pulmonary quantitative analysis of CT images can objectively quantify specific patterns of ILD changes during treatment in patients with systemic sclerosis (SSc)-ILD [48]. The Fleischner Society's recommendation for ILD was that continuous CT scanning of disease is a part of the composite end point of PFT trends and can lead to an area of future research in the multidisciplinary integration of CT and continuous PFT [49]. Recently, the white paper recommendations of the Fleischner Society described the radiologic patterns of IPF and their relationship with the diagnosis of IPF. The imaging patterns (i.e, typical interstitial pneumonia (UIP), probable UIP, indeterminate for UIP, and non-IPF) of UIP were determined by HRCT, and the diagnosis was determined together with clinical pulmonary doctors and the committee of histopathologists [50]. With the right clinical context, for patients with a clear or possible UIP-HRCT pattern, surgical lung biopsy can be abandoned [51]. The severity of tractive bronchiectasis and increased cellulitis have been reported as the strongest determinants of mortality in connective tissue disease related to ILD [52]. The understanding of different modes of IFP, which can identify subtle changes, may be helpful not only to reduce the rate of invasive operations but also to precisely treat patients and evaluate the therapeutic effects [53].
The Application of Radiomics in Bronchial Asthma
Asthma, as a heterogeneous disease, is easily confused with COPD and can benefit from the classification of subtypes. Asthma has been well controlled in a large number of patients, but some patients could not be effectively controlled even if they used high-dose Inhaled Corticosteroids (ICSs) or biological agents. However, radiomics may play an important role in guiding future treatments to improve the recovery of asthma. Improving the prognosis of asthmatic patients by using personalized clinical and imaging biomarkers has been one of the primary goals of the Severe Asthma Research Program (SARP) Project [54]. The quantitative study of CT providing structural and functional information of the lung has been a useful tool for the study of asthma [55-58]. This technique can successfully identify the unique structures and functional phenotypes of asthma and COPD. It was also considered an effective method to distinguish asthma patients and healthy people, as well as COPD patients and asthma patients [59]. Research findings of airway remodelling and air retention in a quantitative study of CT were associated with lung function, asthma severity and histology [60-62]. Studies show that imaging variables, including airway diameter, airway wall thicknesses, and air retention, are important indicators for distinguishing severe asthma from nonsevere asthma. Cluster analysis based on imaging variables is related to clinical features, which can be used to distinguish asthma subgroups and serve as the basis for the development of new therapies [61]. Moreover, Shimss et al. found that potential new airway morphological biomarkers help to understand and monitor local airway remodelling in asthmatic patients. The reduced delta lumen in a subset of subjects with severe, refractory asthma may result in severe clinical outcomes [62]. Importantly, in addition to CT imaging, the use of turbo inversion recovery magnitude sequence functional pulmonary MRI based on radiomics has been shown to accurately detect and evaluate the response of asthmatic patients to treatments. The evaluation of the turbo inversion recovery magnitude sequence can assess the bronchial inflammatory response following segmental allergen challenge in terms of visualization and quantification and show the degree of segmental pulmonary oedema followed by an inflammatory response. This evaluation can provide a promising biomarker for the noninvasive detection of the inflammatory response in asthmatic patients [63]. Furthermore, detection of the percentage of airway wall thickness (wt%), delta lumen and low attenuation area of functional residual volume in a subset of subjects with severe asthma may be useful for the targeted therapy of airway fibrosis and improvement of prognosis for asthma [64]. The progress of radiomics not only is significant in clinical practice but also provides an excellent opportunity to improve decision-making in asthma treatment with more intensive or alternative therapy approaches for individual patients.
The Application of Radiomics in Children's Lung Diseases
Accurate diagnosis has always been a difficult problem in paediatric imaging due to the poor compliance of children, the difficulty of examination and radiation safety. At present, the application of accurate radiomics in children's lung diseases is rare. Only a few studies have focused on semi quantitative CT measurements to quantitatively assess the extent of air trapping [65-68]. Recent studies have demonstrated that the dynamic semi quantitative evaluation of pulmonary perfusion-enhanced imaging can show structural and perfusion abnormalities in children and young people with pulmonary cystic fibrosis and can distinguish operation-related atelectasis from disease-related pulmonary consolidation [69]. Bronchiolitis Obliterans (BO), resulting in damage to the terminal airway and its surroundings, is classified as a potentially irreversible chronic obstructive lung disease with low morbidity and a high mortality rate, including post infectious BO and post transplant BO. The characteristic CT findings in BO include mosaic air retention, bronchiectasis and atelectasis, which are not evenly distributed throughout the lung [65,70]. Quantitative detection of CT in post transplant BO patients with air retention is associated with airway obstruction in PFT [67]. A previous study showed the value of quantitative CT analysis in predicting the severity and longitudinal changes of inhalation lung injury. Quantitative CT analysis could also help to assess pulmonary function by some CT indicators, including Normally Aerated Volume Ratio (NAVR) and Reductively Aerated Volume Ratio (RAVR) [71]. Bronchopulmonary Dysplasia (BPD) is a main cause of premature death and is associated with long-term sequelae in the respiratory system, the neurological system, and cognition in children. Some studies also focused on unique structural abnormalities in chest CT scans of BPD patients and found that the scope of lesions in images correlates with the clinical manifestations and lung function in children with BPD. Unfortunately, data resulting from recent clinical trials were invalid in providing protocols and scoring systems for effectively quantifying structural changes in CT imaging. Therefore, based on the theory and clinical applications of radiomics, CT-based radiomics will serve as a new radiological analysis tool for treatment prediction in lung diseases of children [72].
Furthermore, the study of CT based on imaging in paediatric pulmonary diseases is obviously insufficient compared with that of adult studies. Children are in a period of rapid growth. The physiology and function of the lungs are constantly improving. The lung diseases of children vary from those of adults, and the prognoses of diseases are different. In addition, imaging findings in infants with pulmonary involvement can differ from those in older children and adults. Hence, further studies are needed to more comprehensively define paediatric-specific radiographic findings of lung diseases. Therefore, radiomics has become a challenge in paediatrics, and some problems in clinical application need to be further solved. First, the types of diseases involved in studies were limited, and the sample sizes were small. It is necessary to study multiple diseases and establish large sample data. Second, it is necessary to establish a standardization program for many factors, such as the scanning scheme, research method, parameters, Region Of Interest (ROI) segmentation method, and feature extraction. Third, the stability of the images and the accuracy of radiomics model building also need further study. Last, the relationship between radiomics, histopathology, and genes needs to be further explored in paediatric patients. We have tried to provide a research chart to help clinician and radiologists for development of the CT-based radiomics in lung diseases and application prospects in children (Figure 4).
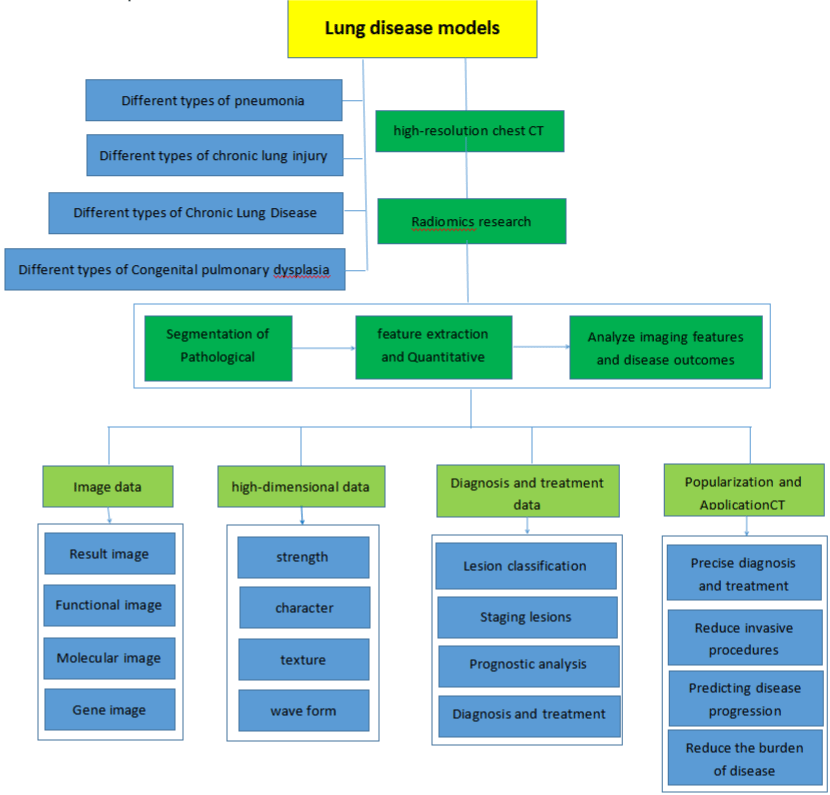
Figure 4: Development of the CT-based radiomics in lung diseases and application prospects in children.
In summary, radiomics can help to identify new biomarkers, provide new insights for understanding the phenotypes of unknown diseases, reduce or avoid traumatic operations, and provide broad prospects for accurate diagnosis and personalized treatment. Unfortunately, at present, radiomics is rarely used in children's lung diseases. Based on the advantages of CT in lung diseases, we aim to focus on CT-based radiomics in lung diseases in children to provide accurate information support for the clinical diagnosis, treatment and differential diagnosis of lung diseases in children and provide a new theory for the realization of accurate medical treatment of lung diseases in children.
Funding
This work was supported by the Jinan Science and Technology Development Plan (202019172), Shandong Provincial Natural Science (ZR2021MH147), and the Health and Family Planning Commission of Jinan Municipalty (2018-1-32).
Acknowledgments
The authors wish to thank study site staff for their cooperation and all the researchers for their efforts in the early stage. We also thank the respiratory departmentand the imaging department of the Jinan Children's Hospital affiliated to Shandong University for the positive suggestions.
References
- Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Stiphout RGPMV, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. European journal of cancer. 2012; 48: 441-446.
- Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Cavalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature Communications. 2014; 5.
- Su HF, Zhou GF, Xie CM, et al. The rise and development of radiomics[J]. Chinese Medical Journal. 2015; 95: 553-556.
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016; 278: 563-577.
- Yongqiang Tan. Computer aided diagnostic methods and performance evaluation of lung CT images. Shanghai Institute of Technology and Physics, China, 2007.
- Zhu Y, Tan Y, Hua Y, Wang M, Zhang G, Zhang J. Feature Selection and Performance Evaluation of Support Vector Machine (SVM)-Based Classifier for Differentiating Benign and Malignant Pulmonary Nodules by Computed Tomography. Journal of Digital Imaging. 2009; 23: 51-65.
- Kido S, Kuriyama K, Higashiyama M, Kasugai T, Kuroda C. Fractal Analysis of Small Peripheral Pulmonary Nodules in Thin-section CT: Evaluation of the Lung-nodule Interfaces. Journal of Computer Assisted Tomography. 2002; 26: 573-578.
- Son JY, Lee HY, Lee KS, Kim J, Han J, Jeong JY, et al. Quantitative CT Analysis of Pulmonary Ground-Glass Opacity Nodules for the Distinction of Invasive Adenocarcinoma from Pre-Invasive or Minimally Invasive Adenocarcinoma. PLoS ONE. 2014; 9: e104066.
- He L, Huang Y, Ma Z, Liang C, Liang C, Liu Z. Effects of contrastenhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule. Scientific Reports. 2016; 6.
- Miwa K, Inubushi M, Wagatsuma K, Nagao M, Murata T, Koyama M, et al. FDG uptake heterogeneity evaluated by fractal analysis improves the differential diagnosis of pulmonary nodules. European journal of radiology. 2014; 83: 715-719.
- Hawkins S, Wang H, Liu Y, Garcia A, Stringfield O, Krewer H, et al. Predicting Malignant Nodules from Screening CT Scans. Journal of Thoracic Oncology. 2016; 11: 2120-2128.
- Vallières M, Freeman CR, Skamene SR, Naqa IE. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Physics in medicine and biology. 2015; 60: 5471-5496.
- Wu W, Parmar C, Grossmann P, Quackenbush J, Lambin P, Bussink J, et al. Exploratory Study to Identify Radiomics Classifiers for Lung Cancer Histology. Frontiers in Oncology. 2016; 6.
- Xue X, Yang Y, Huang Q, et al. Use of a radiomics modelto predict tumor invasiveness of pulmonary adenocarcinomas appearing as pulmonary ground-glass nodules. Biomed Res Int. 2018; 2018: 6803971.
- Yoon HJ, Sohn I, Cho JH, Lee HY, Kim JH, Choi Y, et al. Decoding Tumor Phenotypes for ALK, ROS1, and RET Fusions in Lung Adenocarcinoma Using a Radiomics Approach. Medicine. 2015; 94: e1753.
- Hunter LA, Chen YP, Zhang L, Matney JE, Choi H, Kry SF, et al. NSCLC tumor shrinkage prediction using quantitative image features. Computerized medical imaging and graphics : the official journal of the Computerized Medical Imaging Society. 2016; 49: 29-36.
- Tian Liang,Sheng Xie, a review of Omics Imaging and its application in pulmonary diseases. Journal of China Japan Friendship Hospita. 2019; 33: 35-37.
- Zhang B, Tian J, Dong D, Gu D, Dong Y, Zhang L, et al. RadiomicsFeatures of Multiparametric MRI as Novel Prognostic Factors in Advanced Nasopharyngeal Carcinoma. Clinical Cancer Research. 2017; 23: 4259-4269.
- Delorme S, Keller-Reichenbecher MA, Zuna I, Schlegel W, Kaick GV. Usual interstitial pneumonia. Quantitative assessment of high-resolution computed tomography findings by computer-assisted texture-based image analysis. Investigative radiology. 1997; 32: 566-574.
- WANG Wei, SONG Zu-jun, DING Hong, et al. Imaging studey about distribution sites of lesion areas of pulonary fibrosis induced by paraquat. Chinese Journal of Emergency Medicine. 2011; 6: 614-618.
- Lin Li, Lixin Qin, Zeguo Xu, Youbing Yin, Xin Wang, et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy.Radiology Actions Search in PubMed Search in NLM Catalog Add to Search. 2020; 296: E65-E71.
- Akira M, Atagi S, Kawahara M, Iuchi K, Johkoh T. High-resolution CT findings of diffuse bronchioloalveolar carcinoma in 38 patients. AJR. American journal of roentgenology. 1999; 173: 1623-1629.
- Koo HJ, Kim MY, Koo JH, Sung YS, Jung J, Kim S, et al. Computerized margin and texture analyses for differentiating bacterial pneumonia and invasive mucinous adenocarcinoma presenting as consolidation. PLoS ONE. 2017; 12: e0177379.
- Lu Y, Gong G, Chen J, Qiu Q, Li D, Yin Y. The study of correlation between radiation pneumonitis and the variation of CT-based radiomics features. Chinese Journal of Radiation Oncology. 2018; 27: 643-648.
- Raghunath S, Rajagopalan S, Karwoski RA, et al. Quantitative stratification of diffuse parenchymal lung diseases. PLoS One. 2014; 9: e93229.
- Bartholmai BJ, Raghunath S, Karwoski RA, Moua T, Rajagopalan S, Maldonado F, et al. Quantitative Computed Tomography Imaging of Interstitial Lung Diseases. Journal of Thoracic Imaging. 2013; 28: 298-307.
- Expert Group of Guideline for Primary Care of Respiratory System Disease. Guidline for primary care of chronic obstructive pulmonary disease (2018). Chinese Journal of General Practitioners. 2018; 17: 856-870.
- Madani A, Maertelaer VD, Zanen J, Gevenois PA. Pulmonary emphysema: radiation dose and section thickness at multidetector CT quantification-- comparison with macroscopic and microscopic morphometry. Radiology. 2007; 243: 250-257.
- Hackx M, Bankier AA, Gevenois PA. Chronic obstructive pulmonary disease: CT quantification of airways disease.Radiology. 2012; 265: 34-48.
- Lynch DA, Al-Qaisi MA. Quantitative Computed Tomography in Chronic Obstructive Pulmonary Disease. Journal of Thoracic Imaging. 2013; 28: 284- 290.
- Kauczor H, Wielpütz MO, Owsijewitsch M, Ley-Zaporozhan J. Computed Tomographic Imaging of the Airways in COPD and Asthma. Journal of Thoracic Imaging. 2011; 26: 290-300.
- Lederlin M, Laurent F, Portron Y, Ozier A, Cochet H, Berger P, et al. CT attenuation of the bronchial wall in patients with asthma: comparison with geometric parameters and correlation with function and histologic characteristics. AJR. American journal of roentgenology. 2012; 199: 1226- 1233.
- Montaudon M, Lederlin M, Reich S, Begueret H, Tunon-de-Lara JM, Marthan R, et al. Bronchial measurements in patients with asthma: comparison of quantitative thin-section CT findings with those in healthy subjects and correlation with pathologic findings. Radiology. 2009; 253: 844-853.
- Orlandi I, Moroni C, Camiciottoli G, Bartolucci M, Pistolesi M, Villari N, et al. Chronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuation. Radiology. 2005; 234: 604-610.
- Occhipinti M, Paoletti M, Bartholmai BJ, Rajagopalan S, Karwoski RA, Nardi C, et al. Spirometric assessment of emphysema presence and severity as measured by quantitative CT and CT-based radiomics in COPD. Respiratory Research. 2019; 20.
- Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR. American journal of roentgenology. 2013; 201: W460-W470.
- Lynch DA, Moore CM, Wilson C, Nevrekar D, Jennermann T, et al. CTbased Visual Classification of Emphysema: Association with Mortality in the COPDGene Study. Radiology. 2018; 288: 859-866.
- Grydeland TB, Dirksen A, Coxson HO, Eagan TML, Thorsen E, Pillai SG, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. American journal of respiratory and critical care medicine. 2010; 181: 353-359.
- Han MK, Bartholmai B, Liu LX, Murray S, Curtis JL, Sciurba FC, et al. Clinical Significance of Radiologic Characterizations in COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2009; 6: 459-467.
- Mair G, Maclay J, Miller JJ, McAllister D, Connell M, Murchison JT, et al. Airway dimensions in COPD: relationships with clinical variables. Respiratory medicine. 2010; 104: 1683-1690.
- Lynch DA, Austin JHM, Hogg JC, Grenier PA, Kauczor H, Bankier AA, et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology. 2015; 277: 192-205.
- Occhipinti M, Paoletti M, Bigazzi F, Camiciottoli G, Inchingolo R, Larici AR, et al. Emphysematous and Nonemphysematous Gas Trapping in Chronic Obstructive Pulmonary Disease: Quantitative CT Findings and Pulmonary Function. Radiology. 2018; 287: 683-692.
- Charbonnier J, Pompe E, Moore C, Humphries S, Ginneken BV, Make B, et al. Airway wall thickening on CT: Relation to smoking status and severity of COPD. Respiratory medicine. 2019; 146: 36-41.
- Katherine E Lowe, Elizabeth A Regan, Antonio Anzueto, Erin Austin, John H M Austin, Terri H Beaty, et al. COPDGene® 2019: Redefining the Diagnosis of Chronic Obstructive Pulmonary Disease. Chronic Obstr Pulm Dis. 2019; 6: 384-399.
- Cho MH, Castaldi PJ, Hersh CP, Hobbs BD, Barr RG, Tal-Singer R, et al. A Genome-Wide Association Study of Emphysema and Airway Quantitative Imaging Phenotypes. American journal of respiratory and critical care medicine. 2015; 192: 559-569.
- Boqiang Cai, Yunlong Li, Concord respiratory disease II. China Union Medical University Press, 2011: 2667.
- Tashkin DP, Volkmann ER, Tseng C, Kim HJ, Goldin J, Clements P, et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Annals of the Rheumatic Diseases. 2016; 75: 374-381.
- Occhipinti M, Bosello S, Sisti LG, Cicchetti G, Waure CD, Pirronti T, et al. Quantitative and semi-quantitative computed tomography analysis of interstitial lung disease associated with systemic sclerosis: A longitudinal evaluation of pulmonary parenchyma and vessels. PLoS ONE. 2019; 14.
- Hansell DM, Goldin JG, King TE, Lynch DA, Richeldi L, Wells AU. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials: a position paper from the Fleischner Society. The Lancet. Respiratory medicine. 2015; 3: 483-496.
- Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. The Lancet. Respiratory medicine. 2018; 6: 138-153.
- Funke-Chambour M, Guler SA, Geiser T, Christe A, Heverhagen J, Pöllinger A, et al. New radiological diagnostic criteria: impact on idiopathic pulmonary fibrosis diagnosis. European Respiratory Journal. 2019; 54: 1900905.
- Walsh SL, Sverzellati N, Devaraj A, et al. Connective tissuedisease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. 2014; 69: 216–222.
- Wu X, Kim GH, Salisbury ML, Barber D, Bartholmai BJ, Brown KK, et al. Computed Tomographic Biomarkers in Idiopathic Pulmonary Fibrosis. The Future of Quantitative Analysis. American Journal of Respiratory and Critical Care Medicine. 2019; 199: 12-21.
- Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SAA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. American journal of respiratory and critical care medicine. 2012; 185: 356-362.
- Castro M, Fain SB, Hoffman EA, Gierada DS, Erzurum SC, Wenzel S. Lung imaging in asthmatic patients: the picture is clearer. The Journal of allergy and clinical immunology. 2011; 128: 467-478.
- DeBoer EM, Spielberg DR, Brody AS. Clinical potential for imaging in patients with asthma and other lung disorders. The Journal of Allergy and Clinical Immunology. 2017; 139: 21-28.
- Choi S, Haghighi B, Choi J, Hoffman EA, Comellas AP, Newell JD, et al. Differentiation of quantitative CT imaging phenotypes in asthma versus COPD. BMJ Open Respiratory Research. 2017; 4: e000252.
- Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. American journal of respiratory and critical care medicine. 2000; 162: 1518-1523.
- Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008; 134: 1183-1191.
- Busacker A, Newell JD, Keefe T, Hoffman EA, Granroth JC, Castro M, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009; 135: 48-56.
- Choi S, Hoffman EA, Wenzel SE, Castro M, Fain S, Jarjour N, et al. Quantitative computed tomographic imaging–based clustering differentiates asthmatic subgroups with distinctive clinical phenotypes. The Journal of Allergy and Clinical Immunology. 2017; 140: 690-700.e8.
- Shim SS, Schiebler ML, Evans MD, Jarjour N, Sorkness RL, Denlinger LC, et al. Lumen area change (Delta Lumen) between inspiratory and expiratory multidetector computed tomography as a measure of severe outcomes in asthmatic patients. The Journal of Allergy and Clinical Immunology. 2018; 142: 1773-1780.e9.
- Vogel-Claussen J, Renne J, Hinrichs J, Schönfeld C, Gutberlet M, Schaumann F, et al. Quantification of pulmonary inflammation after segmental allergen challenge using turbo-inversion recovery-magnitude magnetic resonance imaging. American journal of respiratory and critical care medicine. 2014; 189: 650-657.
- Hardy CL, Nguyen H, Mohamud R, Yao J, Oh DY, Plebanski M, et al. The activin A antagonist follistatin inhibits asthmatic airway remodelling. Thorax. 2012; 68: 9-18.
- Mattiello R, Sarria EE, Mallol J, Fischer GB, Mocelin H, Bello R, et al. Post-infectious bronchiolitis obliterans: Can CT scan findings at early age anticipate lung function?. Pediatric Pulmonology. 2010; 45: 315-319.
- Yoon HM, Lee JS, Hwang J, Cho YA, Yoon H, Yu J, et al. Post-infectious bronchiolitis obliterans in children: CT features that predict responsiveness to pulse methylprednisolone. The British journal of radiology. 2015; 88: 20140478.
- Kim HG, Shin HJ, Kim YH, Sohn MH, Lyu CJ, Kim M, et al. Quantitative computed tomography assessment of graft-versus-host disease-related bronchiolitis obliterans in children: A pilot feasibility study. European Radiology. 2015; 25: 2931-2936.
- Kim J, Kim M, Sol IS, Sohn MH, Yoon H, Shin HJ, et al. Quantitative CT and pulmonary function in children with post-infectious bronchiolitis obliterans. PLoS ONE. 2019; 14: e0214647.
- Amaxopoulou C, Gnannt R, Higashigaito K, Jung A, Kellenberger CJ. Structural and perfusion magnetic resonance imaging of the lung in cystic fibrosis. Pediatric Radiology. 2017; 48: 165-175.
- Fischer GB, Sarria EE, Mattiello R, Mocelin HT, Castro-Rodriguez JA. Post infectious bronchiolitis obliterans in children. Paediatric respiratory reviews. 2010; 11: 233-239.
- Ma Y, Zhang S, Zhao L, Zhou X, Mao Z, Xu H, et al. Inhalation lung injury induced by smoke bombs in children: CT manifestations, dynamic evolution features and quantitative analysis. Journal of thoracic disease. 2018; 10: 5860-5869.
- Mastrigt EV, Logie K, Ciet P, Reiss IKM, Duijts L, Pijnenburg MW, et al. Lung CT imaging in patients with bronchopulmonary dysplasia: A systematic review. Pediatric Pulmonology. 2016; 51: 975-986.