
Review Article
Austin J Anat. 2022; 9(1): 1107.
Lymphangiogenesis and Prostanoids as Regulators in Disease States
Majima M1,2*, Hosono K2, Ito Y2, Amano H2, Nagashima Y1,3, Matsuda Y4, Watanabe S5 and Nishimura H6
1Department of Medical Therapeutics, Kanagawa Institute of Technology, Japan
2Department of Pharmacology, Kitasato University School of Medicine and Department of Molecular Pharmacology, Japan
3Tokyo Research Laboratories, Kao Corporation, Japan
4Department of Life Support Engineering, Kanagawa Institute of Technology, Japan
5Department of Exercise Physiology and Health Sciences, Kanagawa Institute of Technology, Japan
6Department of Biological Information, Faculty of Health and Medical Sciences, Kanagawa Institute of Technology, Japan
*Corresponding author: Masataka Majima, Department of Pharmacology and Medical Therapeutics, Kanagawa Institute of Technology & Kanagawa Institute of Technology, Japan
Received: August 19, 2022; Accepted: August 30, 2022; Published: September 06, 2022
Abstract
The lymphatic vessels have crucial roles in the regulation of interstitial fluids, immune surveillance and the absorption of dietary fat in the intestine. Lymphatic functions are also closely related to the pathogenesis of various disease states such as inflammatory responses, lymphedema, and metastasis of tumors. Lymphangiogenesis, the formation of new lymphatic vessels from pre-existing lymphatics is critical determinant of the above pathological conditions. Prostanoids including prostaglandins and thromboxanes are the metabolites of arachidonic acid, C-20 unsaturated fatty acid, and they exhibit a variety of actions via specific receptors. Although some kinds of growth factors are well-characterized in the lymphangiogenesis, there is accumulating evidence that prostaglandinsand thromboxanesare important regulators of lymphangiogenesis. Previously prostanoids are reported to have immediate actions on the smooth muscles and vasculatures, however, they work as inducers of growth factors or cytokines that regulate lymphangiogenesis, and modulate the prolymphangiogenic microenvironment. This review discusses our current understanding of prostanoids as regulators of lymphangiogenesis, and the emerging importance of the lymphangiogenesis as a therapeutic target.
Keywords: Lymphangiogenesis; Inflammation; Lymphedema; Metastasis; Prostaglandin; Thromboxane
Introduction
Blood vessel system is a closed circulatory one with microcirculation in every organ. By contrast, lymphatic vessel system is a one-way conduit for tissue fluids and leukocytes including lymphocytes [1]. The critical function of lymphatic vessels is to drain the protein-rich fluid extravasated from the blood vessels and to drive it back into the systemic circulation [1,41]. Lymphatics exhibit significant roles in immune surveillance, and deliver various antigens and antigen-presenting cells to the regional lymph nodes and export immune effector cells and humoral response factors into the circulation. Dysfunction of lymphatics induce lymph accumulation in interstitial spaces, and tissue swellings, lymphedema. Lymphatic functions exhibit major determinant of pathogenesis of various disease states such as inflammatory responses (Schwager et al, 2019), lymphedema (Zheng et al, 2014), endometriosis (Hattori et al, 2020), liver dysfunctions (Nakamoto et al, 2020), and metastasis of tumor cells (Dieterich et al, 2022).
Biologically active lipids including prostanoids exhibit various activities on the cardiovascular systems. Recent evidence indicates that biologically active lipids regulate lymphangiogenesis, similar to angiogenesis, under certain pathological conditions [21,43]. During chronic inflammation, angiogenesis is induced by a variety of inflammatory mediators, such as prostaglandins [40]. However, the accumulated knowledge relevant to bioactive lipid-associated lymphangiogenesis is limited. It was recently clarified the roles of cyclooxygenase-2 and prostaglandinE2 [21,42] receptor signaling in enhancement of lymphangiogenesis during chronic and proliferative inflammation [21,42]. The same machinery was also active in the secondary lymphedema model, in which lymphedema was induced by a circumferential incision made in the tail of anesthetized mice to sever the dermal lymphatic vessels [29]. This review article summaries current knowledge on prostanoids relevant to pathological lymphangiogenesis. The receptor signaling and/or biosynthesis of prostanoids will be a promising target to treat the various pathological states related to lymphangiogenesis.
Biosynthesis and Receptors of Prostanoids
Prostanoids including prostaglandins and thromboxanes are the metabolites of arachidonic acid, C-20 unsaturated fatty acid [31]. Prostaglandins contain a cyclopentane ring with two attached side chains named a and ω; a side chain has terminal carboxylic acid. According to the modification of the cyclopentane ring, physiologically important prostaglandins are classified into 4 types: PGD2, PGE2, PGF2a and PGI2. ThromboxaneA2 (TXA2), another prostanoid, has an oxane ring instead of the cyclopentane ring. Arachidonic acids are released from membrane phospholipids with various physiological and pathological stimuli by the action of phospholipase A2. Arachidonic acids are converted to various prostanoids by sequential actions of cyclooxygenases and the respective prostanoid synthases. There are 2 isoforms of cyclooxygenases, cyclooxygenases-1 and cyclooxygenases-2 [31]. Cyclooxygenases-1 is a constitutive isoform and has housekeeping functions, whereas cyclooxygenases-2 is an inducible isoform and has a proinflammatory nature. PGH2, a common precursor of prostanoids that were produced by cyclooxygenases, is converted to each prostanoid via the action of respective prostaglandin synthase or thromboxane synthase [31]. The expression profile of these synthases vary depending on cell types or tissues.
Prostanoids exhibit their activities acting on the receptors specific for PGD, PGE, PGF, PGI and TX, namely DP, EP, FP, IP and TP receptors. EP receptors are classified into four subtypes, EP1, EP2, EP3 and EP4. Thromboxane A2receptor (TP) was first purified from human blood platelets. Homology screening in mouse cDNA libraries subsequently identified the structures of all of the eight types and subtypes of the prostanoid receptors, which belong to a G-protein-coupled receptor super family [39,48]. The most typical actions of prostanoidsare relaxation and contraction of various types of smooth muscles. According to these actions of prostanoids on smooth muscles, the classification of the prostanoid receptors has been proposed (Coleman et al., 1994). The contractile receptors, EP1, FP and TP, couple to Gq and raise intracellular Ca2+concentrations. These receptors couple to G12/13 and activate Rho, a low molecular weight GTP-binding protein that organizes cytoskeleton. By contrast, the relaxant receptors, DP, EP2, EP4 and IP, couple to Gs and raise intracellular cyclic adenosine monophosphate (cAMP) concentrations. Interestingly EP4 has another signaling pathway related to phosphatidylinositol 3-kinase, activating protein kinase B and extracellular signal-regulated kinases [12]. In contrast, the inhibitory receptor EP3 couples mainly to Giand decreases intracellular cAMP concentrations, while several EP3 isoforms derived from alternative splicing have other signaling pathways [47].
Highly selective agonists and antagonists had not been developed, thus it had been very difficult to evaluate the functions of each prostanoid receptor to the pathogenesis. However, mouse lacking each prostanoid receptor individually or enzyme mediating prostanoid biosynthesis have been generated by gene targeting, and significances of the prostanoid actions in various pathophysiological processes are being tested [3,21,43]. This review summarizes the current knowledge on roles of prostanoids in the pathogenesis of lymphangiognesisassociated disease conditions. The field of prostanoids is rich with the candidates of therapeutic tools.
Lymphangiogenesis and Prostanoids Relevant to Disease States
Prostanoids regulate Lymphangiogenesis in Inflammation
Under physiological conditions, the plasma is filtered through the semipermeable vascular endothelial layer into the extracellular space. The majority of the extravasated interstitial fluid and macromolecules are absorbed back by the lymphatic vessels (Levick and Michel, 2010). A primary function of the lymphatic system is to provide an accessory return route for the interstitial fluids to the blood (Levick, 2004). When inflammation was sustained, drainage of the interstitial fluid into the circulation was increased together with antigens and immune cells from the periphery to lymph nodes with which adaptive immune responses are operated [33]. Lymphangiogenisis appeared to be critical since applications of prolymphangiogenic factor, vascular endothelial growth factor (VEGF)-C reduced the process of skin inflammation [22,25].
In LPS-induced inflammation, it was demonstrated that cyclooxugenase-2-derived prostaglandins especially PGE2 enhanced inflammation-induced lymphangiogenesis (InfL) [42]. InfL may modulate the pathological environment by influencing fluid movement and immunological functions. Inflammation is an intrinsically beneficial event that leads to the removal of harmful factors and restoration of tissue structure and physiological function. Once the noxious stimulus is removed, the inflammatory reaction decreases and resolves. It had been demonstrated previously that in proliferative inflammation, prostaglandin receptor signaling elicited adenylate cyclase-induced angiogenesis [2,3]. Prostaglandins induced vascular endothelial growth factor-A (VEGF-A) in the granulation tissues formed during inflammatory responses. The same pathway was shown to be active in the tumor microenvironment [30]. Among the cyclooxugenase-2-derived metabolites of arachidonic acid, PGE2 is the most abundantly generated metabolite, and mediates an array of proinflammatory activities [31,39].
In lipopolysaccharide (LPS)-induced peritonitis, the number of LYVE-1-positive ladder-structured lymphatics in the diaphragm increased temporally [42]. This lymphangiogenic response was accompanied by increased expression of VEGF-C/D in the inflamed tissues. In mice treated with celecoxib, a cyclooxygenase-2 inhibitor, InfL was suppressed with reduced expression of VEGF-C/D. This was also observed in microsomal PGE2 synthase-1 (mPGES-1) knockout mice. mPGES-1 is an inducible perinuclear enzyme that is functionally linked with cyclooxygenase-2. Cyclooxygenase-2 and mPGES-1are essential components for PGE2 synthesis, which may be linked to inflammation, fever, osteogenesis, and cancer. Immunoreactive cyclooxygenase-2 and mPGES-1 were detected in both CD11b-positive and CD3ε-positivecells in the diaphragm. When FITC-dextran was injected into the peritoneal cavities, the amount of residual FITC-dextran was reduced significantly in WT mice injected with LPS, and this reduction was significantly decreased in mPGES-1 KO mice [42].
In a separate experiment, it was clarified that when cultured lymphatic endothelial cells were incubated with pathophysiological concentration of PGE2 (1 nM and 10 nM), they did not proliferate markedly with no induction of VEGF-A and VEGF-C. By contrast, PGE2 (1 nM and 10 nM) markedly induced VEGF-A and VEGF-C in harvested peritoneal macrophages (Kashiwagi et al., 2014). These results suggested that cyclooxygenase-2/mPGES-1-derived PGE2 played a significant role in lymphangiogenesis during inflammation, and represents a novel target for controlling InfL. PGE2 may act as aninducers for VEGF isoforms acting not on lymphatic endothelial cells, but on the inflammatory cells accumulated in the prolymphangiogenicmicroenvironment (Kashiwagi et al., 2014).
Lymphangiogenesis is also related to the progression of Inflammatory Bowel Disease (IBD). Blockade of VEGF receptor-3 (VEGFR-3) aggravates inflammatory bowel disease and lymphatic vessel enlargement [23]. Recently it is demonstrated [20] that lymphangiogenesis contributes to mucosal repair in acute colitis elicited by Dextran Sulphate Sodium (DSS) [7,32]. EP4 signaling suppresses the development of this experimental colitis contributing to lymphangiogenesis, in turn promoting mucosal tissue repair. Compared with vehicle, treatment with EP4 antagonist increased signs of colitis, colonic tissue destruction, and CD11b+ cell infiltration, with reduced area of lymphatic vessels. By contrast, an EP4 agonist treatment suppressed the severity colitis with suppressed CD11b+ infiltration and decreased expression levels of inflammatory cytokines. These changes were associated with upregulation of prolymphangiogenic growth factors and lymphangiogenesis. Importantly, inhibition of VEGFR-3signaling delayed mucosal repair, accompanied with impaired lymphangiogenesis. These results suggest that EP4 stimulation enhances mucosal repair in DSS-induced acute colitis by promoting lymphangiogenesis [32].
Inflammation often persists and becomes chronic. Chronic inflammation is characterized by the persistent infiltration of mononuclear cells including macrophages and lymphocytes, and fibrocytes [37]. Lymphagiogenesisis observed in the process of chronic inflammation. Growing evidence suggests involvement of chronic inflammatory processes in pathogenesis of a variety of diseases including cancer [62]. In such a disorder, abundant infiltration of inflammatory cells and expression of various pro-inflammatory molecules are found in affected tissues. Substantial evidence has now accumulated indicating that PGs contribute to lymphangiogenesis during the process of chronic inflammation [21].
In a granulation tissue formed around the matrigels injected subcutaneously, which exhibited proliferative inflammation profiles and mimicked tumor microenvironment, lymphangiogenesis was enhanced by PGE2 [21]. During chronic inflammation in the surgical sponge implants, angiogenesis is induced by a variety of inflammatory mediators including prostaglandins [2,3,40,49,61,66] Majima et al., 1997. It had been demonstrated that cyclooxygenase-2 and PGE2 receptor signaling enhanced lymphangiogenesis during proliferative inflammation formed around matrigels [21]. Lymphangiogenesis estimated by podoplanin/VEGFR-3/LYVE-1 expression was upregulated during proliferative inflammation seen around Matrigel plugs. A cyclooxygenase-2 inhibitor (celecoxib) reduced lymphangiogenesis, whereas topical PGE2 application enhanced lymphangiogenesis. Lymphatic flow from the Matrigels was cyclooxygenase-2 dependent. Lymphangiogenesis was suppressed in the granulation tissues of mice lacking either EP3 or EP4, suggesting that these molecules are receptors in response to endogenous PGE2. An EP3-selective agonist increased the expression of VEGF-C and VEGF-D in cultured macrophages, whereas an EP4-selective agonist increased VEGF-C expression in cultured macrophages and increased VEGF-D expression in cultured fibroblasts [21]. This study suggested that cyclooxugenase-2 and EP3/EP4 signaling contributes to lymphangiogenesis via VEGF-C and VEGF-D, and may become a therapeutic target for controlling lymphangiogenesis in chronic inflammation (Figure 1).
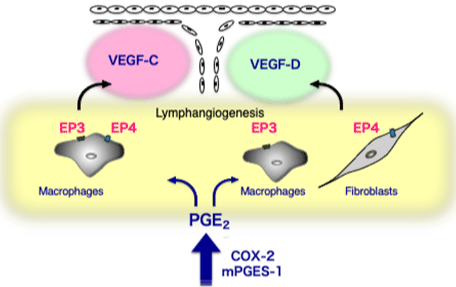
Figure 1: EP receptor signaling relevant to the enhancement of
lymphangiogenesis in chronic inflammation.
COX-2/mPGES-1-derived PGE2 stimulated EP3/EP4 receptors and
upregulated VEGF-C and VEGF-D in fibroblasts and macrophages. As
discussed, prostanoids act as an inducer of growth factors in the inflammatory
sites.COX; cyclooxygenase, mPGES-1; microsomal PGE2 synthase-1,
VEGF; vascular endothelial growth factor. (Cited from Arterioscler Thromb
Vasc Biol. 2011; 31: 1049-58. with permission).
Recently, an unexpected metabolite of arachidonic acid was reported to inducelymphangiogenesis in the pathological conditions [43]. TXA2 is an unstable metabolite of arachidonic acid, produced by cyclooxygenase and thromboxane synthase catalysis in various cell types, and exerts its activity through a TP [48]. The roles of TXA2 in cardiovascular system responses related to ischemic heart disease and brain hemorrhage, as well as platelet aggregation and bronchial smooth muscle contraction [48], are well-understood; however, it was found that TXA2-TP signaling also modulates acquired immunity by regulating dendritic cell T-cell interactions [24]. These reports provide a new avenue for exploration of the pathophysiological roles of TXA2 in immunity, particularly in the lymph system. TP signaling on the platelets was reported to enhance the angiogenesis via platelet adhesion to angiogenic vasculatures [2,5,44]. Platelets supplied proangiogenic factors during the adhesion process. Further, TPdependent platelet adhesion in the liver microvasculatures restored liver damages [45,53].
The peritoneum provides the lining of the peritoneal cavity and is the largest serous membrane in the body (Gandawidjaja and Hau, 1997). A single layer of mesothelial cells covers the peritoneal cavities. A thin and discontinuous layer of connective tissues and a layer of fenestrated lymphatic vessels are present under the mesothelial cells [60]. These 3 layers function as an absorptive site for peritoneal fluids [15,18]. Once inflammation was induced in the peritoneal cavities, the fluid reabsorption was highly dependent to the lymphatic function. To analyze the functional relevance of lymphatics to the fluid reabsorption, the diaphragm is a suitable organ [34].
In the peritonitis model, a novel function of thromboxane, the facilitation of lymphangiogenesis via TP signaling was reported using TP knockout mice [43]. Compared with wild-type mice, LPS-induced lymphangiogenesis in systemic TP-knockout mouse diaphragm tissues was suppressed with reduced drainage function from the peritoneal cavity. TP-positive macrophages and T cells accumulated in the diaphragm produced VEGF-C and VEGF-D in a TP-dependent manner. Removal of macrophages and T cells resulted in reduced lymphangiogenesis and lowered expressions of VEGF-C and VEGF-D. Furthermore, TP knockout bone marrow chimeric mice exhibited reduced lymphangiogenesis. When cell-specific TP knockout using TP flox mice (Tp F/F) were tested [43], TP knockout specific to macrophages and T cells led to reduced lymphangiogenesis and drainage function in mice with LPS injections. This study suggested that TP signaling exerts prolymphangiogenic activity by acting on macrophages and T cells and that TP signaling represents a novel target for controlling lymphangiogenesis (Figure 2).
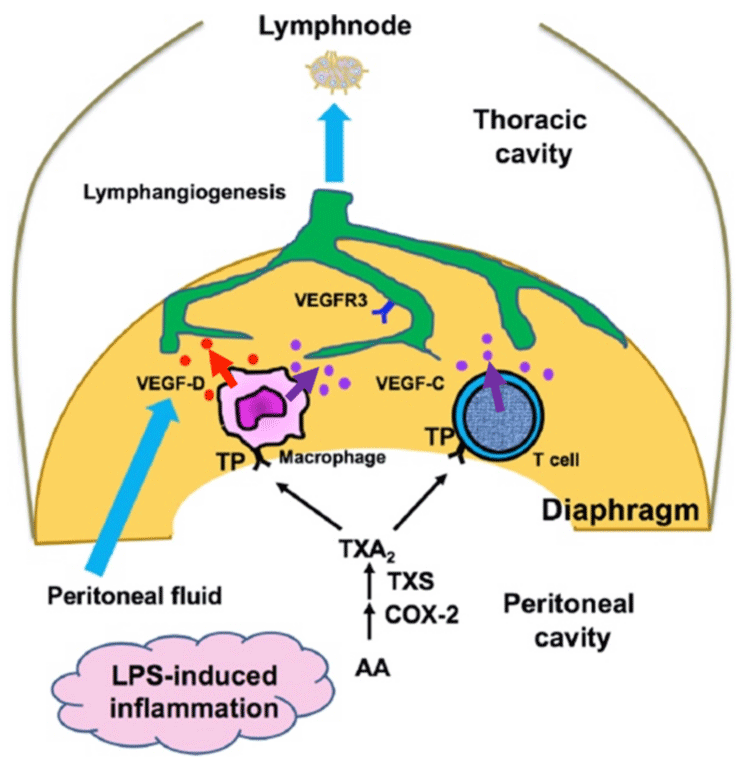
Figure 2: TP signaling in macrophages and T cells enhances
lymphangiogenesis with upregulation of VEGF-C and VEGF-D expression in
LPS-induced inflammation.
Results from the systemic knockout (KO) of TP, together with macrophagespecific/
T cell-specific KO, suggest that TP on macrophages and T cells
facilitates the generation of functionally active lymphatic vessels in the
diaphragm. TP; Thromboxane A2 receptor. (Cited from Arterioscler Thromb
Vasc Biol. 2021; 41: 1390-1407. with permission).
Prostanoids regulate Lymphagiogenesis in Lymphedema
The lymphatic vasculature forms a network of vessels that drain interstitial fluid from tissues and return it to the blood [1]. Surgical treatment of malignant tumors, such as lymphadenectomy in patients with breast cancer, sometimes induces lymphedema that can impact quality of life [11]. The present therapeutic approaches for secondary lymphedema patients are the physical therapies such as a combination of compression, exercise, and massage. When physical therapy is not effective, super microsurgical techniques to make the anastomosis of blood vessels and lymphatic vessels is applied. Although the induction of lymphangiogenesis may be a possible therapeutic approach, it is not clear to what extent therapies aimed at lymphatic regrowth will be effective for the treatment of various types of secondary lymphedema. If lymphedema is attributable to blockade of lymph flow such as surgical removal of lymph nodes, therapies that stimulate lymphangiogenesis to restore lymphatic flow may be a good option for the treatment of secondary lymphedema.
Key regulators in lymphatic development have been demonstrated by transgenic models [52]. VEGFR-3 signaling may be responsible for primary lymphedema [27]. The recent findings suggest that targeting of molecular mechanisms of lymphangiogenesis to improve the lymphatic function may lead to a successful treatment of lymphedema by enhancing the recurrence of lymphatic flow in the secondary lymphedema situation.To clarify the roles of cyclooxygenase-2 in enhancement of lymphangiogenesis during secondary lymphedema, a mouse tail model had been tested [29]. The diameters of the tails around the wounds were markedly increased after surgery, and reached maximum size 2 weeks after wounding in mice without celecoxib. Expression of cyclooxygenase-2 in wound granulation tissues was markedly increased 1 week after surgery compared with unwounded naive control mice. In mice without celecoxib, recurrence of lymphatic flow in the wound granulation tissues was detected 3 weeks after surgical treatment. In contrast, lymphatic flow was markedly suppressed in mice treated with celecoxib. Newly formed lymphatic structures were identified in the granulation tissues formed at wounded lesions, whereas those were markedly suppressed with celecoxib. Interstitial tissue pressures in the distal areas of the tail wounds were markedly increased in mouse with celecoxib with reduced expression of VEGF-C. This study suggested that lymphangiogenesis, together with recurrence of lymph flow after surgical induction of lymphedema, was upregulated by cyclooxygenase-2 via generation of prostaglandins [29].
Prostanoids regulate tumor - Associated Lymphnagiogenesis and Lymph Node Metastasis
It had been repeatedly reported that prostaglandins facilitated tumor-associated angiogenesis especially acting on the stromal tissues [63,66]. It was revealed that prostaglandins enhanced lymphangiogensis in tumor microenvironment [36]. Prostaglandins are involved in a range of cellular functions, however, it had been emphasized that prostaglandins act as an inducer of VEGF isoforms with prolymphangiogenic potentials [21]. The importance of prostaglandin-mediated dilation during metastasis was demonstrated in an animal model of lymphogenous spread, in which it was shown that the lymphangiogenic factors VEGF-C and VEGF-D were able to induce dilation of collecting lymphatic vessels draining the primary tumor mass, leading to increased metastatic load in the sentinel lymph node [28]. Tumor studies conducted in EP1-4 receptor knockout mice or using pharmacological inhibition revealed that these receptors have a role in promoting tumorigenesis, angiogenesis and lymphangiogenesis in various tumor settings and therefore provide attractive targets for therapeutic intervention [3,36].
In tumor microenvironment, PGE2 and E Preceptor signaling pathways have been implicated in the promotion of tumor growth, however, little is known about their roles in lymphangiogenesis during tumor development. A previous study clarified that endogenous PGE2 exhibited a critical role in tumor-associated lymphangiogenesis [36]. Treatment of mice with celecoxib, down-regulated the expression of VEGFR-3 in stromal tissues markedly, and attenuated expression of podoplanin, a marker for lymphatic endothelial cells. EP3 receptor knockout mice exhibited a significant reduction in tumor weight associated with a reduction in VEGFR-3 mRNA expression in tumor stromal tissues [36]. This result suggested that host EP3 receptor signaling regulates tumor-associated lymphangiogenesis by upregulating expression of VEGF-C and its receptor, VEGFR-3, in tumor stromal tissues. Host EP3 blockade together with cyclooxygenase-2 inhibition may be a novel therapeutic strategy to suppress tumorassociated lymphangiogenesis.
Patients with advanced cancers have a significant risk of local recurrence and distant metastasis after surgical resections of the primary tumors. It is widely accepted that many tumors tend to metastasize to specific organs [55]. The mechanisms that guide tumor cells to specific tissues are largely unknown, although recent evidence suggests that it may involve molecular differences inherent in the tumor cells themselves, modulated by the activities of immune cells, hematopoietic cells, and other tissue components. Lymph Node Metastasis (LNM) is a critical prognostic factor in cancer patients, and lymphatic vessels serve as an important route for the spread of cancer cells [55]. Paget reported that tumor cells might prepare the lymph nodes for their future arrival, giving a new interpretation to the seed-and-soil hypothesis [54]. The formation of a premetastatic niche, suitable for the arrival of the first tumor cells, facilitates metastasis via the bloodstream [16,17,26]. However, data regarding the factors involved in lymph node premetastatic niche formation are limited [35]. Tumor-associated lymphangiogenesis may enhance metastasis to the regional lymph nodes; however, the involvement of lymph node premetastatic niche formation in the metastatic process is not fully clarified. Among the many cellular components within the tumor microenvironment, Dendritic Cells (DCs) exert profound effects on T cells [64]. The mediators that regulate DC function may also modulate niche formation. PGE2 and the tryptophan-catabolizing enzyme Indoleamine 2,3-Dioxygenase (IDO) exert strong effects on the maturation and function of DCs [8]. PGE2 has been identified as a major immunosuppressive soluble factor present in the tumor microenvironment [58]. An important mechanism by which these DCs modulate T cell responses seems to be via PGE2-induced expression of IDO. Furthermore, a recent study reported that PGE2 increased the immunosuppressive potential of regulatory T cells (Tregs) [59]. It had been previously reported that cyclooxygenase-2–derived endogenous PGE2 enhanced angiogenesis and lymphangiogenesis during tumor development and chronic inflammation [3,21,36]. Furthermore, PGE2 enhances stromal tissue formation and tumor-associated angiogenesis mediated by tumor stromal chemokines [30]. The function of a broad range of immune cells can be regulated by PGE2; however, the reports on the precise contributions of PGE2 to LNM are limited.
In a study using EP knockout mice, it had been shown that endogenous cyclooxygenase-2–derived PGE2 stimulated the EP3 receptor on DCs and upregulated the expression of stromal cell– derived factor-1 (SDF-1) in the subcapsular regions of regional lymph nodes following Lewis Lung Carcinoma (LLC) cell injection [50]. SDF-1 upregulation increased the accumulation of CXCR4+ LLC cells and facilitated the formation of regional lymph node premetastatic niches. The accumulation of Tregs and lymph node lymphangiogenesis, both of which may influence the fate of metastasized tumor cells, were also cyclooxygenase-2/EP3–dependent. Thus, inhibitors of PGE2, together with SDF-1 receptor antagonists and EP3 antagonists, may be effective at suppressing premetastatic niche formation and LNM. These findings strongly suggested a critical function of PGE2 in the formation of the lymph node premetastatic niche [50] (Figure 3). In a separate experiment, Aspirin had been shown to reduce the metastasis of lung carcinoma cells to the regional lymph nodes, with a significant reduction in mortality [51]. In rodent models of breast cancer, cyclooxygenase-2 inhibition decreased mammary tumorassociated lymphangiogenesis, tumor cell invasion into lymphatics, and metastasis [38].
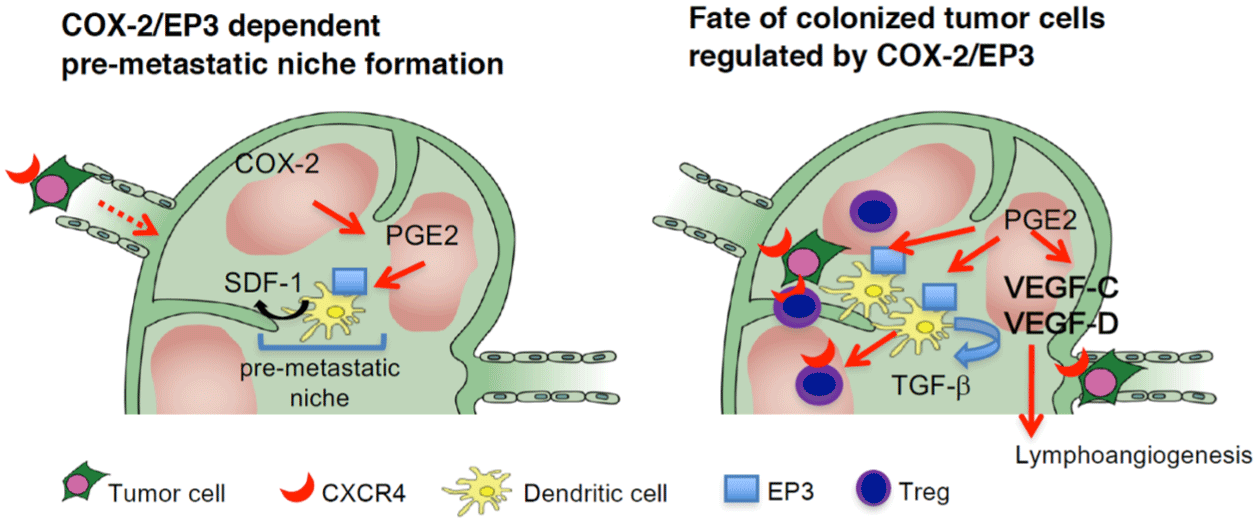
Figure 3: COX-2/EP3-dependent premetastatic niche formation in a reginal lymph node.
COX-2 and EP3 mediate the increase of SDF-1 at the premetastatic site before the arrival of tumor cells to the regional lymph node. CXCR4-positive tumor cells
were mobilized to SDF-1–generating sites. Simultaneously, CXCR4/EP3–positive DCs are recruited to generate SDF-1 in response to EP3 signaling. Tregs are
recruited to the premetastatic niche via SDF-1/EP3 signaling. DCs recruited to the premetastatic niche secrete TGF-β1 in an EP3/SDF-1–dependent manner.
COX-2/PGE2-EP3 signaling together with SDF-1/CXCR4 signaling may decide the fate of the colonizing LLC cells. COX-2/EP3 signaling upregulates lymph
node lymphangiogenesis in the efferent side of the subcapsular region, and this facilitates tumor cell growth in the regional lymph nodes and lymph node or
systemic metastasis.COX; cyclooxygenase, CXCR; CXC chemokine receptor, SDF; stromal cell-derived factor.(Cited from J. Clin. Invest. 2014; 124: 4882-94. with
permission).
Key Findings and Conclusions
Prostanoids exhibit various activities via G-coupled receptors. PGE2 and TXA2 are representative molecules that induce the constrictions of various smooth muscles, hypotension, and platelet activation/inhibition. These actions are quite immediate ones, however accumulated results indicate that prostanoids also induce angiogenesis/lymphangiogenesis via up-regulations of growth factors such as VGEF-A, -C, and -D, and cytokines such as SDF-1 [2,6,21]. As discussed in each section of this review, EP3 and EP4 have prolymphangiogenic activities. Although EP3 couples mainly to Gi and decreases intracellular cAMP concentrations, several EP3 isoforms derived from alternative splicing have other signaling pathways [47]. It is reported previously that a variant of EP3 couples to adenylate cyclase/protein kinase A to induce VEGF-A [2]. EP3- mediated increase in cAMP may facilitate lymphnagiogenesis in inflammation and tumor microenvironment [21,36]. Further, EP4 couples to Gs and raises intracellular cAMP concentrations. A rise in the second messenger, cAMP in the cytosol via activation of adenylate cyclase activates Protein Kinase A (PKA), and PKA in turn activates a transcription factor CREB (cAMP response element-binding protein). Although the promoter region of VEGF-C gene contains binding sites for Sp1, AP-2 and NF-kB [9], CREB that belongs to the basic leucine-zipper family and binds to a specific regulatory element in the promoters of the target genes enhances VEGF-C expression [57]. Accumulated results suggest that the prostaglandin receptors that increase cAMP concentrations may activate gene expressions of VEGF-C/D.
Further, it is well-established that TP communicates mainly with Gq and G13, resulting in phospholipase C activation and RhoGEF activation, respectively [65], however TP couples with G11, G12, G13, G14, G15, G16, Gi, Gs and Gh too [46]. Gsa subunit family which stimulates adenylate cyclase [14,56] may stimulate lymphangiogenesis as observed in the LPS-induced peritonitis [43]. This TP activity may be promising to treat pathological conditions including inflammation.
As discussed, gate keepers of the lymphangiogenic process have emerged as a novel class of therapeutic targets. Several antiangiogenic antibodies were applied clinically, however, clinical trials targeted to lymphangiogenesis are very limited. We had summarized present knowledge on prostanoids to induce lymphangiogenesis. PGE2 has a prolymphangiogenic activities. Interestingly, it does not act on LECs rather on the inflammatory cells derived from the bone marrow in some models. Thus, it exhibits a landscaping effect in the microenvironments during inflammation. Agonists for EP3 and EP4 may become a stimulator of lymphangiogenesis in vivo [19,21]. Application of the agonists of these receptors to lymphedema patients will be promising. Biosynthesis of PGE2 in pathological settings is dependent on cyclooxygenase-2 and mPGES-1. Further, together with inhibitors for cyclooxygenase-2 and mPGES-1, antagonists for EP3 and EP4 will be effective tool to block lymphangiogenesis in some kinds of models [36,42]. Interplay between the immune cells and prostanoids appears important in other pathological conditions related to the lymphatic plasticity [43,50]. Interestingly, inflammationinduced lymphangiogenesis may blocked by TP inhibition on T cells and macrophages [43], and lymph node metastasis may be blocked via cyclooxygenase-2/mPGES-1/EP3 inhibitions on DCs [50,51]. These suggest that the field of the arachidonic acid metabolites is quite rich with therapeutic candidates. As discussed, prostanoids act as a transcriptional factor to induce VEGF isoforms and other cytokines. Prostanoids act beyond the concepts of the classical activities as smooth muscle contracting substances and vasoactive substances.
Author Contributions
All authors have been equally involved in writing and editing the manuscript. Figures have been done by KH and MM. All authors contributed equally to the article and approved the submitted version.
Funding
This work was supported by grants from The Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, no. 22K06870 (MM), and from Takeda Science Foundation (MM), Uehara Memorial Foundation (MM), and a grant, no. 26311616 from Kanagawa Institute of Technology (MM).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alitalo K. The lymphatic vasculature in disease. Nature Medicine. 2011; 17: 1371-1380.
- Amano H, Ando K, Minamida S, Hayashi I, Ogino M, Yamashina S, et al. Adenylate cyclase/protein kinase A signaling pathway enhances angiogenesis through induction of vascular endothelial growth factor in vivo. Japanese journal of pharmacology. 2001; 87: 181-188.
- Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, et al. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003; 197: 221-232.
- Amano H, Ito Y, Eshima K, Kato S, Ogawa F, Hosono K, et al. Thromboxane A2 induces blood flow recovery via platelet adhesion to ischaemic regions. Cardiovascular research. 2015; 107: 509-521.
- Amano H, Nakamura M, Ito Y, Kakutani H, Eshima K, Kitasato H, et al. Thromboxane A synthase enhances blood flow recovery from hindlimb ischemia. The Journal of surgical research. 2016; 204: 153-163.
- Amano H, Eshima K, Ito Y, Nakamura M, Kitasato H, et al. The microsomal prostaglandin E synthase-1/PGE2 axis induces recovery from ischemia via recruitment of regulatory T cells. Cardiovasc Res. 2022; 20: cvac137.
- Betto T, Amano H, Ito Y, Eshima K, Yoshida T, Matsui Y, et al. Vascular endothelial growth factor receptor 1 tyrosine kinase signaling facilitates healing of DSS-induced colitis by accumulation of Tregs in ulcer area. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019; 111: 131-141.
- Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005; 106: 2375-2381.
- Chilov D, Kukk E, Taira S, Jeltsch M, Kaukonen J, Palotie A, et al. Genomic Organization of Human and Mouse Genes for Vascular Endothelial Growth Factor C*. The Journal of Biological Chemistry. 1997; 272: 25176-25183.
- Coleman RA, Grix SP, Head SA, Louttit JB, Mallett A, Sheldrick RL. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994; 47: 151-168.
- Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. Journal of the National Cancer Institute. 2001; 93: 96-111.
- Fujino H, West KA, Regan JW. Phosphorylation of Glycogen Synthase Kinase-3 and Stimulation of T-cell Factor Signaling following Activation of EP2 and EP4 Prostanoid Receptors by Prostaglandin E2 *. The Journal of Biological Chemistry. 2002; 277: 2614-2619.
- Maula E, Kasanen E. Cher Fermat A.D. 1637. Philosophica. 1989;43. doi:10.21825/philosophica.82455
- Giannarelli C, Zafar MU, Badimon JJ. Prostanoid and TP-receptors in atherothrombosis: is there a role for their antagonism?. Thrombosis and haemostasis. 2010; 104: 949-954.
- Hall JC, Heel KA, Papadimitriou JM, Platell C. The pathobiology of peritonitis. Gastroenterology. 1998; 114: 185-196.
- Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. The Journal of Experimental Medicine. 2005; 201: 1089-1099.
- Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, et al. The S100A8–serum amyloid A3–TLR4 paracrine cascade establishes a pre-metastatic phase. Nature Cell Biology. 2008; 10: 1349- 1355.
- Ho H, Wu M, Yang Y. Peritoneal Cellular Immunity and Endometriosis. American Journal of Reproductive Immunology. 1997; 38: 400-412.
- Hosono K, Isonaka R, Kawakami T, Narumiya S, Majima M. Signaling of Prostaglandin E Receptors, EP3 and EP4 Facilitates Wound Healing and Lymphangiogenesis with Enhanced Recruitment of M2 Macrophages in Mice. PLoS ONE. 2016; 11: e0162532.
- Hosono K, Kojo K, Narumiya S, Majima M, Ito Y. Prostaglandin E receptor EP4 stimulates lymphangiogenesis to promote mucosal healing during DSS-induced colitis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2020; 128: 110264.
- Hosono K, Suzuki T, Tamaki H, Sakagami H, Hayashi I, Narumiya S, et al. Roles of Prostaglandin E2–EP3/EP4 Receptor Signaling in the Enhancement of Lymphangiogenesis During Fibroblast Growth Factor-2–Induced Granulation Formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011; 31: 1049-1058.
- Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011; 117: 4667-4678.
- Jurisic G, Sundberg JP, Detmar M. Blockade of VEGF Receptor-3 Aggravates Inflammatory Bowel Disease and Lymphatic Vessel Enlargement. Inflammatory Bowel Diseases. 2013; 19: 1.
- Kabashima K, Murata T, Tanaka H, Matsuoka T, Sakata D, Yoshida N, et al. Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nature Immunology. 2003; 4: 694-701.
- Kajiya K, Sawane M, Huggenberger R, Detmar M. Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin inflammation by promoting lymphangiogenesis. The Journal of investigative dermatology. 2009; 129: 1292-1298.
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche. Nature. 2005; 438: 820-827.
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, et al. A model for gene therapy of human hereditary lymphedema. Proceedings of the National Academy of Sciences of the United States of America. 2001; 98: 12677-12682.
- Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer cell. 2012; 21: 181-195.
- Kashiwagi S, Hosono K, Suzuki T, Takeda A, Uchinuma E, Majima M. Role of COX-2 in lymphangiogenesis and restoration of lymphatic flow in secondary lymphedema. Laboratory Investigation. 2011; 91: 1314-1325.
- Katoh H, Hosono K, Ito Y, Suzuki T, Ogawa Y, Kubo H, et al. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. The American journal of pathology. 2010; 176: 1469-1483.
- Katori M, Majima M. Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflammation Research. 2000; 49: 367- 392.
- Kawashima-Takeda N, Ito Y, Nishizawa N, Kawashima R, Tanaka K, Tsujikawa K, et al. RAMP1 suppresses mucosal injury from dextran sodium sulfate-induced colitis in mice. Journal of Gastroenterology and Hepatology. 2017; 32: 809-818.
- Kim H, Kataru RP, Koh GY. Inflammation-associated lymphangiogenesis: a double-edged sword?. The Journal of clinical investigation. 2014; 124: 936- 942.
- Kim KE, Koh Y, Jeon B, Jang C, Han J, Kataru RP, et al. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. The American journal of pathology. 2009; 175: 1733-1745.
- Kim M, Koh YJ, Kim KE, Koh BI, Nam D, Alitalo K, et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer research. 2010; 70: 10411-10421.
- Kubo H, Hosono K, Suzuki T, Ogawa Y, Kato H, Kamata H, et al. Host prostaglandin EP3 receptor signaling relevant to tumor-associated lymphangiogenesis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2010; 64: 101-106.
- Kumar V, Abbas A and Aster J (2018) Robbins Basic Pathology 10th Edition. Elsevier: 81–95.
- Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, et al. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. The Journal of clinical investigation. 2014; 124: 3901-3912.
- Majima M, Amano H, Hayashi I. Prostanoid receptor signaling relevant to tumor growth and angiogenesis. Trends in pharmacological sciences. 2003; 24: 524-529.
- Majima M, Hayashi I, Muramatsu M, Katada J, Yamashina S, Katori M. Cyclooxygenase- 2 enhances basic fibroblast growth factor-induced angiogenesis through induction of vascular endothelial growth factor in rat sponge implants. British Journal of Pharmacology. 2000; 130: 641-649.
- Majima M, Hosono K, Ito Y, Amano H. Biologically active lipids in the regulation of lymphangiogenesis in disease states. Pharmacology & therapeutics. 2021; 232: 108011.
- Matsuda H, Hosono K, Tsuru S, Kurashige C, Sekiguchi K, Akira S, et al. Roles of mPGES-1, an inducible prostaglandin E synthase, in enhancement of LPS-induced lymphangiogenesis in a mouse peritonitis model. Life sciences. 2015; 142: 1-7.
- Matsuda H, Ito Y, Hosono K, Tsuru S, Inoue T, Nakamoto S, et al. Roles of Thromboxane Receptor Signaling in Enhancement of Lipopolysaccharide- Induced Lymphangiogenesis and Lymphatic Drainage Function in Diaphragm. Arteriosclerosis, Thrombosis, and Vascular Biology. 2021; 41: 1390-1407.
- Matsui Y, Amano H, Ito Y, Eshima K, Suzuki T, Ogawa F, et al. Thromboxane A2 receptor signaling facilitates tumor colonization through P-selectinmediated interaction of tumor cells with platelets and endothelial cells. Cancer Science. 2012; 103: 700-707.
- Minamino T, Ito Y, Ohkubo H, Shimuzu Y, Kojo K, Nishizwa N, et al. Adhesion of platelets through thromboxane A receptor signaling facilitates liver repair during acute chemical-induced hepatotoxicity. Life sciences. 2015; 132: 85- 92.
- Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacology & therapeutics. 2008; 118: 18-35.
- Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, et al. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993; 365: 166-170.
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiological reviews. 1999; 79: 1193-1226.
- Oba K, Hosono K, Amano H, Okizaki S, Ito Y, Shichiri M, et al. Downregulation of the proangiogenic prostaglandin E receptor EP3 and reduced angiogenesis in a mouse model of diabetes mellitus. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2014; 68: 1125-1133.
- Ogawa F, Amano H, Eshima K, Ito Y, Matsui Y, Hosono K, et al. Prostanoid induces premetastatic niche in regional lymph nodes. The Journal of clinical investigation. 2014; 124: 4882-4894.
- Ogawa F, Amano H, Ito Y, Matsui Y, Hosono K, Kitasato H, et al. Aspirin reduces lung cancer metastasis to regional lymph nodes. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2014; 68: 79-86.
- Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annual review of cell and developmental biology. 2005; 21: 457-483.
- Otaka F, Ito Y, Goto T, Eshima K, Amano H, Koizumi W, et al. Platelets prevent the development of monocrotaline-induced liver injury in mice. Toxicology letters. 2020; 335: 71-81.
- Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8:98-101. PMID: 2673568.
- Pepper MS. Lymphangiogenesis and tumor metastasis: myth or reality?. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001; 7: 462-8.
- Reilly M, Fitzgerald GA. Cellular activation by thromboxane A2 and other eicosanoids. Eur Heart J. 1993 Dec;14 Suppl K:88-93. PMID: 8131796.
- Singh NK, Kotla S, Kumar R, Rao GN. Cyclic AMP Response Element Binding Protein Mediates Pathological Retinal Neovascularization via Modulating DLL4-NOTCH1 Signaling. EBioMedicine. 2015; 2: 1767-1784.
- Sombroek CC, Stam AGM, Masterson AJ, Lougheed SM, Schakel MJAG, Meijer CJLM, et al. Prostanoids Play a Major Role in the Primary Tumor- Induced Inhibition of Dendritic Cell Differentiation. The Journal of Immunology. 2002; 168: 4333-4343.
- Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat J, et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood. 2013; 122: 1419-1427.
- Tsilibary EC, Wissig SL. Light and electron microscope observations of the lymphatic drainage units of the peritoneal cavity of rodents. The American journal of anatomy. 1987; 180: 195-207.
- Ueno T, Suzuki T, Oikawa A, Hosono K, Kosaka Y, Amano H, et al. Recruited bone marrow cells expressing the EP3 prostaglandin E receptor subtype enhance angiogenesis during chronic inflammation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2010; 64: 93-100.
- Wang D, DuBois RN. An Inflammatory Mediator, Prostaglandin E2, in Colorectal Cancer. The Cancer Journal. 2013; 19: 502-510.
- Williams CS, Tsujii M, Reese J, Dey SK, Du Bois RN. Host cyclooxygenase-2 modulates carcinoma growth. The Journal of clinical investigation. 2000; 105: 1589-1594.
- Wilson NS, Villadangos JA. Regulation of antigen presentation and crosspresentation in the dendritic cell network: facts, hypothesis, and immunological implications. Advances in immunology. 2005; 86: 241-305.
- Woodward DF, Jones RL, Narumiya S. International Union of Basic and Clinical Pharmacology. LXXXIII: Classification of Prostanoid Receptors, Updating 15 Years of Progress. Pharmacological Reviews. 2011; 63: 471- 538.
- S, Amano H, Hayashi I, Kitasato H, Kamata M, Inukai M, et al. COX-2/ VEGF-Dependent Facilitation of Tumor-Associated Angiogenesis and Tumor Growth in vivo. Laboratory Investigation. 2003; 83: 1385-1394.