
Review Article
Austin J Anat. 2022; 9(1): 1108.
POEMS Syndrome-Clinical Picture and Management
Artur Jurczyszyn¹, Magdalena Olszewska-Szopa²* and David Vesole³
1Department of Hematology, Jagiellonian University Medical College, 31-501 Kraków, Poland
2Department of Hematology, Blood Neoplasms and Bone Marrow Transplantation, Wroclaw Medical University, Wroclaw, Poland
3Hackensack University Medical Center, New Jersey Medical School, Rutgers University, Hackensack, NJ, USA
*Corresponding author: Magdalena Olszewska-Szopa, Department of Hematology, Blood Neoplasms and Bone Marrow Transplantation, Wroclaw Medical University, Wroclaw, Poland
Received: September 27, 2022; Accepted: October 26, 2022; Published: November 02, 2022
Introduction
The term ‘POEMS syndrome’ was created in 1980 [1]. The acronym derived from words: polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes, does not cover the variety of symptoms that can be observed in this disease.
Keywords: POEMS, plasma cell dyscrasia, therapy
Pathophysiology and Epidemiology
One of the most important factors in POEMS mechanism seems to be overproduction of pro inflammatory cytokines eg. Vascular Endothelial Growth Factor (VEGF), tumor necrosis factor-a (TNF- -a) and interleukin -6 (IL-6). High levels of these cytokines are responsible for symptoms like edema and effusions in the mechanism of endothelial permeability increase. The disease is actually considered a paraneoplastic phenomenon secondary to plasma cell dyscrasia [2]. Little is known about cytogenetic aspect of the disease. Presence of a small plasmacytic clone makes research problematic [3]. Chen at al analyzed the genetic profile of bone marrow cells in 42 POEMS patients, finding high mutational heterogeneity [4]. In an earlier publication by Nagao et al., based on the molecular analysis of 20 patients, the authors suggested a distinct genetic POEMS plasma cells profile from MGUS and MM cells [5]. The differences in the clinical characteristics of the studied patients and the diagnostic methods do not allow for easy comparison of the two above-mentioned reports.
Although the exact incidence and prevalence is unknown, POEMS is declared to be a rare condition. The first and major challenge in making the diagnosis is coming up with an idea that POEMS might be the explanation of patients problems.
Diagnostic criteria are listed in Table 1.
MANDATORY
(both required)
- Clonal plasma cell dyscrasia
- Polyneuropathy
MAJOR
(1 of 3 required)
- Castleman disease
- VEGF elevation
- Osteosclerotic lesions
MINOR
(1 of 6 required)
- Organomegaly: spleno/hepatomegaly, lymphadenopathy
- EndocrinoptahyA
- Extravascular volume overload: oedema, pleural effusion, ascites
- Skin changes: hyperpigmentation,hypertrichosis, glomeruloid hemangiomata, plethora, acrocyanosis, flushing, white nails
- Papilledema
- Polycythemia/Thrombocytosis
A: Diabetes mellitus or thyroid abnormality alone-does not fulfill minor criterion, VEGF: Vascular Endothelial Growth Factor.
Table 1: POEMS syndrome diagnostic criteria according to Barwick, 1980 [1].
Mandatory Criteria
Monoclonal Plasma Cell Disorder (PCD) detection is essential for crucial role of monoclonal plasma cells in the disease pathophysiology. Plasma Cell Myeloma (PCM) or Waldenstrom Macroglobulinemia are revealed occasionally, although the criteria for these diseases are not fulfilled in many cases. Blood and/or urine tests should reveal monoclonal protein presence. Trephine biopsy, more sensitive than bone marrow smear, shows plasma cells in two thirds of patients, at least 95% of which are lambda restricted [6,7]. The average plasma cell percentage does not exceed 5% [2].
Another mandatory criterion is peripheral neuropathy. Ascending symmetric sensory or less often motor symptoms usually occur primarily in distal parts of extremities. They might not be prominent. If POEMS is suspected, whereas neuropathy symptoms are not evident, neurological tests should be conducted. Electromyography reveals an abated nerve conduction, delayed distal latencies and compound muscle action potential [8]. Nerve biopsy, rarely performed, shows demyelination, often with axonal loss, and uncompacted myelin lamellae [9]. The mechanism of POEMS neuropathy is still not well understood though some assumptions about potential mechanisms were published [10].
Major Criteria
Castleman disease. POEMS associated multicentric Castleman disease (POEMS associated MCD -multicentric disease) is distinguished from other MCDs [11]. Histologically often mixed or plasmacytic variant are observed. Castleman variant of POEMS is characterized by a milder neuropathy than in typical form of the disease [12].
VEGF is a sensitive and specific marker in POEMS diagnosis [13]. Although probably not a driving force in POEMS, VEGF is considered the best prognostic marker [14]. The growth of VEGF correlates best with the activity of the disease [15]. This cytokine affects endothelial cells which lead to an increase in its permeability [2]. It is worth mentioning serum levels are 10-50 times higher than plasma levels [16]. When assessing VEGF level one should be aware of the fact, that corticosteroid therapy might profoundly lower the result [2]. Other cytokines, eg Il-12, Il-6, IL-1β, have been shown to play a role in POEMS pathogenesis [2,17]. A correlation between Il- 12 level and disease activity was also observed [18].
Novel markers for POEMS have been reported in recent years. Wang et al, in 2014, proposed N-terminal propeptide of type I collagen (P1NP)[19]. In 2020 Isshiki et al described osteopontin, encoded by SPP1 gene, elevation in POEMS patients [20].
Osteosclerotic lesions are revealed in 95% patients in plain radiological tests. However additional presence of osteolysis is often observed. In more than 50% cases the lesions are numerous [8]. Variable fluorodeoxyglucose uptake is seen in PET-CT [21,22], however PET-CT and low dose CT give the benefit of visualizing possible organomegaly and as cites [2].
Minor Criteria
Skinchanges. A wide range of abnormalities are considered POEMS syndrome connected: hyperpigmentation, hypertrichosis, glomeruloid hemangiomata, plethora, acrocyanosis, flushing, clubbing, white nails. Even in normal appearing skin biopsy might reveal vascular abnormalities in histopathological examination [23].
Extravascular overload. Peripheral oedemas are most characteristic, but fluid might accumulate in peritoneum, pleura and pericardium.
Endocrine disturbances apply to thyroid, parathyroid, adrenal functioning, diabetes mellitus and hypogonadism. Thyroid function abnormalities and diabetes mellitus due to its high prevalence cannot be considered criteria for POEMS [2]. Gonadal axis abnormalities are most commonly observed endocrine symptoms [2,24].
Papilledema. Elevated cytokine production leads to increased vascular permeability and optic disc swelling. On the basis of 94 patients analysis Cui et al considered papilledema an independent negative prognostic factor in POEMS [25].
Organomegaly. Hepatomegalyas well as splenomegaly might be present. Lymphadenopathy, usually mild, may be a part of Castleman disease [26].
Polycythemia/Thrombocytosis. Increased number of platelets and/or hemoglobin level is describeda common laboratory feature in POEMS syndrome. However 26% POEMS patients were anemicin Li study [27].
Diagnostic and clinical features with differences in frequencies given by distinct authors are listed in Table 2.
Feature
Dispenzieri
2019[12]Zhao
2019[48]Wang
2017[70]Jurczyszyn
2022[46]Number of patients
metaanalysis
347
362
108
Age
-
>50 y - 40%
>50y -40%
median age 51
Male sex (%)
-
65,7
62
72
Polycythemia (%)
12-19
15
Thrombocytosis (%)
54-88
51
38
M protein on serum electrophoresis
24-54
BMPC >10% (%)
-
3,7
3
Castleman disease (%)
11-25
61,5
64
23
Splenomegaly (%)
Hepatomegaly (%)
Lymphadenopathy (%)22-70
24-78
26-7459
38
6467
47
6544
47
46Any endocrinopathy (%)
Gonadal axis abnonrmalities67-84
55-89
>68
58
31Bone lesions present (%)
27-97
=>55
Skin lesions (%)
68-89
>87
69
Extravascular volume overload (%)
29-87
84
>87
62
Papilledema
29-64
39
Weight loss (%)
37
50
Fatigue (%)
31
67
BMPC: Bone Marrow Plasma Cell Percentage
Table 2: Clinical and laboratory features in POEMS. Differences in frequencies given by distinct authors.
In more than 90% patients creatinine level is within normal limits and significant proteinuria is present in less than 10% cases. But cystatin-C is elevated in 71% patients [2]. Pulmonary involvement may vary in severity in POEMS: restrictive lung disease, pulmonary hypertension, reduced diffusion capacity of carbon monoxide. Pulmonary hypertension was diagnosed in 25 [28] to 27% [29] POEMS patients.
Thrombotic Complications
Based on small studies arterial and venous thrombosis is claimed to appear in 20% of POEMS patients [12]. Therapy regimens used in POEMS, adapted from plasma cell myeloma, such as immunomodulating drugs have prothrombotic effect [2]. However, thrombotic risk in myeloma seems to be lower than in POEMS [30]. Sayar et al analyzed retrospectively data from 83 patients from UK POEMS registry. They revealed that 30% of POEMS cohort experienced arterial and/or venous thrombotic event. According to their observations arterial thrombosis was more common than venous (16 vs 18 cases respectively), and the highest risk period preceded therapy beginning [31]. Based on these results University College London Hospitals (UCLH) recommend concurrently prophylactic LMWH and single antiplatelet drug until serum VEGF drops below 1000 pg/mL. If immunomodulating agent is used USLH advises to maintain prophylactic LMWH [31]. Mellors et al analyzed retrospectively the data of 230 POEMS patients. The rate of thrombotic complications was 27% and, as in the report by Sayar et al, the predominance of arterial thrombosis was observed. Among the risk factors for arterial thrombosis, the authors mentioned thrombocytosis, high hematocrit, extravascular volume overload and splenomegaly, and for venous thrombosis hyperprolactinemia only. Recurrent disease was observed in 21% of thrombotic patients [32].
Feng et al analyzed retrospectively 510 POEMS patients. Up to 8% of them suffered from Ischemic Stroke (IS), which occurred mainly within the first three months after the diagnosis. Furthermore recurrent IS were documented in 37% of ischemic patients, but not in those in haematological remission [33]. Mellors at al reported similar IS rates (7%) [32].
Prognosis and Risk Factors
The number of clinical features present at diagnosis does not determine survival in POEMS [8].
Kourelis et al based on the retrospective analysis of 291 POEMS patients, determined younger age (RR 0.98 [0.96-1.00]), albumin greater than 3.2 g/dL (RR 0.5 [0.32-0.89]) and attainment ofcomplete hematologic response (RR 0.4 [0.2-0.9]) good prognostic factors. These parameters affect RR (relapse rate) and OS (overall survival) [39].
According to Yang observations based on 383 POEMS cases retrospective analysis, baseline hypothyroidism is an independent prognostic factor for OS (82.8 vs. 92.8%) and PFS (68.9 vs. 82.5%) [40].
Extravascular volume overload [8], pulmonary disorders [41] and ischemic complications [Feng et al] are associated with unfavorable prognosis in POEMS.
Castleman disease presence might negatively influence OS in POEMS patients [2, 31].
Prognostic factors of potential value are listed in Table 3.
Negative prognostic factor
author
Positive prognostic factor
author
Age >50
Wang 2017[70]
Complete hematological response
Kourelis 2016[34]
Pleural effusion
Wang 2017[70]
Albumin >=3,2 g/dl
Kourelis 2016[34]
eGFR < 30 mL/min/1.73 m2
Wang 2017[70]
Low VEGF level
Scarlato 2005[10]
Pulmonary hypertension
Wang 2017[70]
Ischemic stroke
Feng 2020[33]; Fu 2017[71]
Thrombosis
Mellors 2022[32]
Early relapse
Kourelis 2016[34]
Baseline hypothyroidism
Yang 2020[72]
Castleman disease presence
Dispenzieri 2019[12]
Li 2011[27]Papilledema
Cui 2014[25]
eGFR: Glomerular Filtration Rate; VEGF: Vascular Endothelial Cell Growth Factor.
Table 3: Negative and positive prognostic factors in POEMS according to various authors.
Therapy
The course of treatment is primarily established on the bone disease extent and bone marrow involvement [42]. Therapy scheme is presented in Figure1.
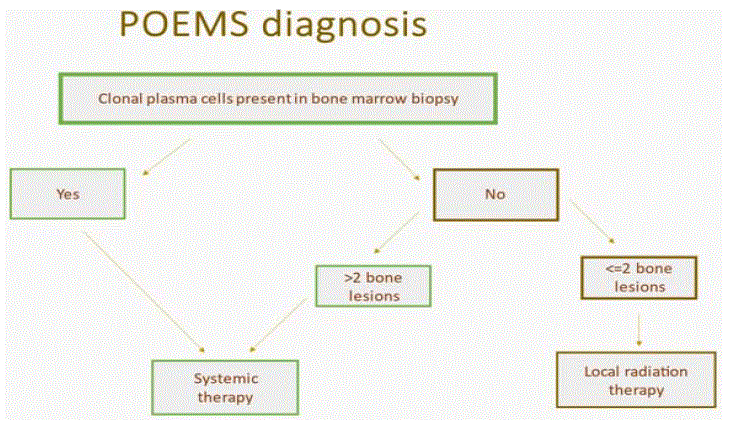
Figure 1: Therapeutic algorithm in POEMS.
Limited Stage Disease
Cases with absence of clonal plasma cells in bone marrow biopsy and 1-3 lesions are considered limited stage disease. These patients are treated with radiation therapy at a dose of 35-50 Gy. In Mayo Clinic experience, published in 2013, 4 year OS was 97% and event free survival 52% respectively [43].
Advanced Disease
Due to the rarity of the disease there are no guidelines for therapy in POEMS, and myeloma therapeutic schedules are regularly implemented.
Neurologic response is usually seen no sooner than after 6 months after treatment implementation, one has to wait for 2-3 years for maximal effect. The lag between therapy and other symptoms relief might be smaller. In case of PET-CT response it takes 6-12 months to illustrate the effect [2].
Although clinical response means quality of life improvement, it is hematological remission that influences patient’s outcome. The longest PFS is observed in patients achieving hematological CR with 6 year PFS - 88% [39].
High dose melphalan and authological stem cell transplantation (HDT/AHCT).
Autologous stem cell transplantation allows to achieve significant clinical improvement in all surviving POEMS patients [39]. Patients may undergo this procedure either upfront or after preceding induction therapy [40]. According to the International Blood and Marrow Transplant Research (CIBMTR) database, ASCT is performed with no previous therapy in 22% and after induction treatment in 70% [41]. Cook et al analyzed 127 HDT/AHCT patients reported in European Group for Blood and Marrow Transplantation (EBMT) database. Hematologic response was available in 101 cases, with 48% of HRCR (N=49) [42].Tomkins at al analyzed 42 HDT/AHCT cases in the care of UCLH. They identified 33,3 % complete hematological responses HRCR, and 16,7% very good partial responses HRVGPR. Clinical response was achieved in 86% of patients. 5-year OS was seen in 89,5% [43]. Other data support these observations [39,44,45,46]. Conditioning regimen is adopted from myeloma-melphalan in doses within the range of 140-200mg/m² [39,47]. HDT/ASCT was more effective than lenalidomide plus dexamethason (Lendex) and considerably more efficient than alkeran plus prednisone (MP) in a large retrospective study of Zhao et al [48]. However it should be mentioned that lowest risk patients were in ASCT cohort in this analysis.
Second ASCT in relapsed patients is a feasible option in selected cases [49,50].
Although the differences in OS between ASCT and non-ASCT patients decrease with the wider use of novel agents, future studies to assess the usefulness of ASCT in this indication are necessary [51].
Low dose alkylator-based therapy. Even in the era of novel drugs long term alkylating therapy remains an option. Prospective clinical trial documented at least partial symptom relief in 100% and hematological response in 81% patients [52].
Immunomodulating agents are used in induction treatment as well as in relapsed/refractory disease. Due to its neurotoxicity thalidomide is not recommended, but the role of lenalidomide still grows. Lenalidomide is usually introduced in doublets with dexamethasone. In a prospective II phase study Li et al demonstrated 46% complete haematologic responses and 95% neurologic responses with 3-year OS 90% and 3 year PFS 75% in response to lenalidomide/dexamethasone combination [53]. Other publications with prostective trials [54] and retrospective analyses [55] support these results.
Proteasome inhibitors. Bortezomib use is considered an effective drug in POEMS. Combinations with dexamethasone and occasionally cyclophosphamide are employed. According to published data bortezomib induces quick symptomatic improvement. Gao et al described a population of 69 patients treated with Veldex (bortezomib, dexamethasone). After a median of 9 cycles 46,4% (N=32) achieved CRH, while 88,1% (N=52)experienced a marked neurological improvement. The authors reported reversible bortezomib induced neuropathy only in 2 patient. After a median follow up of 22,5 months estimated 2-year OS was 95,7% [56]. He and colleges analyzed 20 cases of POEMS treated with VCD (bortezomib, cyclophosphamide, dexamethasone). Bortezomib dose was decreased to 1mg/m2. 41,2% of patients achieved CRH, 95% experienced neurological improvement. No grades => 3 side effects were observed during therapy [57]. Early observations on a small group of 11 patients treated with ixazomib in combination with lenalidomide and dexamethasone are encouraging [58].
There are only single reports on the use of daratumumab in POEMS [59,60,61,62].
Future Therapies
The data on CART based therapy in POEMS is sparse. There are single publications, eg on anti BCMA therapy in a patient with POEMS and myeloma [63].
The data on anti VEGF therapy are conflicting [64]. It has shown benefits in several cases [65,66], but has failed in a few more [67,68].
Before therapy starting it is important to differentiate between PCM and POEMS. Even though therapeutic palette is similar in both cases, potential toxicity of myeloma therapy might be higher in POEMS which influences decision.
Response Criteria and Monitoring
Given how small the clone in POEMS is, monitoring the response also poses a challenge to the clinician. VEGF is not an ideal disease marker, light chains ratio may remain normal for a long time despite the free light chains increase [69]. The selected response criteria are listed in Table 4. Additionally, various organ response scales are used depending on the initial advancement of the disease.
feature/aspect
Complete response
Improvement
Progression
Heamotologic
Negative: immunofixation of blood and urine;
bone marrow assesment= 50% M-spike reduction
= 25% M-spike increase from the lowest level
(at least 0,5 g/dl)VEGF
Negative
= 50% reduction
= 50% increase from the lowest level
PET-CT
Negative
= 50% reduction
in uptake value= 30% increase
in uptake value2021 Update on diagnosis, risk-stratification, and management [2].
M-spike - monoclonal protein spike; VEGF - vascular endothelial growth factor; PET-CT-positron emission tomography - computed tomography
Table 4: Selected response criteria in POEMS. based on Dispenzieri A. POEMS syndrome:
References
- Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, et al. Plasma cell dyscrasia withpolyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore). 1980; 59: 331-322.
- Dispenzieri A. POEMS syndrome: 2021 Update on diagnosis, riskstratification, and management. Am J Hematol. 2021; 96: 872-888.
- Wang C. POEMS syndrome remains a mystery after 40 years. Leukemia. 2021; 35:1220-1221.
- Chen J, Gao XM, Zhao H, Cai H, Zhang L, et al. A highly heterogeneous mutational pattern in POEMS syndrome. Leukemia. 2020; 35: 1100-1107.
- Nagao Y, Mimura N, Takeda J. Yoshida K, Shiozawa Y, et al. Genetic and transcriptional landscape of plasma cells in POEMS syndrome. Leukemia. 2019; 33: 1723-1735.
- Aravamudan B, Tong C, Lacy MQ, Hayman SR, Buadi F, et al. Immunoglobulin variable light chain restriction, cytokine expression and plasma cell-stromal cell interactions in POEMS syndrome patients. ASH Annual Meeting Abstracts. 2008;112: 2744.
- Wang C, Huang XF, Cai QQ, Cao XX, Cai H, et al. Remarkable expression of vascular endothelial growth factor in bone marrow plasma cells of patients with POEMS syndrome. Leuk Res. 2016; 50: 78-84.
- Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TM, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003; 101: 2496.
- Sung JY, Kuwabara S, Ogawara K, Kanai K, Hattori T, et al. Patterns of nerve conduction abnormalities in POEMS syndrome. Muscle Nerve. 2002; 26: 189.
- Scarlato M, Previtali SC, Carpo M, Pareyson D, Briani C, et al. Polyneuropathy in POEMS syndrome: role of angiogenic factors in the pathogenesis. Brain. 2005; 128: 1911-1920.
- Fajgenbaum DC, Uldrick TS, Bagg A, Frank D, Wu D, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/ idiopathic multicentric Castleman disease. Blood. 2017; 129: 1646-1657.
- Dispenzieri A. POEMS Syndrome: 2019 Update on diagnosis, riskstratification, and management. Am J Hematol. 2019; 94: 812-827.
- Pihan M, Keddie S, D'Sa S, Church AJ, Yong KL, et al. Raised VEGF: High sensitivity and specificity in the diagnosis of POEMS syndrome. Neurol Neuroimmunol Neuroinflamm. 2018; 5: e486.
- Zhao H, Cai H, Wang C, Huang XF, et al. Prognostic value of serum vascular endothelial growth factor and hematological responses in patients with newlydiagnosed POEMS syndrome. Blood Cancer J. 2018; 8: 37.
- D'Souza A, Hayman SR, Buadi F, Mauermann M, Lacy MQ, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood. 2011; 118: 4663-4665.
- Tokashiki T, Hashiguchi T, Arimura K, Eiraku N, Maruyama I, et al. Predictive value of serial platelet count and VEGF determination for the management of DIC in the crow-Fukase (POEMS) syndrome. Intern Med. 2003; 42: 1240- 1243.
- Soubrier M, Dubost JJ, Serre AF, Ristori JM, Sauvezie B, et al. Growth factors in POEMS syndrome: evidence for a marked increase in circulating vascular endothelial growth factor. Arthritis Rheum. 1997; 40: 786-787.
- Kanai K, Sawai S, Sogawa K, Mori M, Misawa S, et al. Markedly upregulated serum interleukin-12 as a novel biomarker in POEMS syndrome. Neurology. 2012; 79: 575-582.
- Wang C, Zhou YL, Cai H, Cheng X-Q, Zhang W, et al. Markedly elevated serum total N-terminal propeptide of type I collagen is a novel marker for the diagnosis and follow up of patients with POEMS syndrome. Haematologica. 2014; 99: e78-e80.
- Isshiki Y, Mimura N, Oshima M, Kayamori K, Seki M, Nagai Y. Single-cell analyses unravel unique features of plasma cell clones in POEMS syndrome. In Proceedings of the ASH Annual Meeting. 2020.
- Albertí MA, Martinez-Yélamos S, Fernandez A, Vidaller A, Narváez JA, et al. 18F-FDG PET/CT in the evaluation of POEMS syndrome. Eur J Radiol. 2010; 76: 180-182.
- Montoriol PF, Cachin F, Michel JL, Soubrier M, et al. Two more cases of evaluation of POEMS syndrome using 18-FDG PET/CT. Eur J Radiol. 2011; 80: 861-864.
- Santoro L, Manganelli F, Bruno R, Nolano M, Provitera V, et al. Sural nerve and epidermal vascular abnormalities in a case of POEMS syndrome. Eur J Neurol. 2006; 13: 99-102.
- Yang H, Zhao H, Gao X, Huang X, Cao X, et al. Endocrine Evaluation in POEMS Syndrome: A Cohort Study. Front. Endocrinol. 2020; 11: 536241.
- Cui R, Yu S, Huang X, Zhang J, Tian C, et al. Papilloedema is an independent prognostic factor for POEMS syndrome. J Neurol. 2014; 261: 60-65.
- Shi XF, Hu SD, Wu LL, Chen X-Y, Wu J-N, et al . Lymphadenopathy in POEMS syndrome: a correlation between clinical features and imaging findings. Int J Clin Exp Pathol. 2020; 13: 21-25.
- Li J, Zhou DB, Huang Z, Jiao L, Duan M-H, et al. Clinical characteristics and long-term outcome of patients with POEMS syndrome in China. Ann Hematol. 2011; 90: 819-826.
- Lesprit P, Godeau B, Authier FJ, Soubrier M, Zuber M, et al. Pulmonary hypertension in POEMS syndrome: a new feature mediated by cytokines. Am J Respir Crit Care Med. 1998; 157: 907-911.
- Li J, Tian Z, Zheng HY, Zhang W, Duan M-H, et al. Pulmonary hypertension in POEMS syndrome. Haematologica. 2013; 98: 393-398.
- Bradbury CA, Jenner MW, Striha A, Cook G, Pawlyn C, et al. Thrombotic events in patients with myeloma treated with immunomodulatory drugs; results of the Myeloma XI study. Blood. 2017; 130: 553.
- Sayar Z, Weatherill A, Keddie S, Sive J, Lunn MP, et al. High rates of venous and arterial thrombotic events in patients with POEMS syndrome: results from the UCLH (UK) POEMS registry. Blood Adv. 2020; 4: 2139-2142.
- Mellors PW, Kourelis T, Go RS, Muchtar E, et al. Characteristics and risk factors for thrombosis in POEMS syndrome: A retrospective evaluation of 230 patients. Am J Hematol. 2022; 97: 209-215.
- Feng J, Gao XM, Zhao H, He T-H, Zhang CL, et al. Ischemic stroke in patients with POEMS syndrome. Blood Adv. 2020; 4: 3427-3434.
- Kourelis TV, Buadi FK, Kumar SK, Gertz MA, Lacy MQ, et al. Risk factors for and outcomes of patients with POEMS syndrome who experience progression after first-line treatment. Leukemia. 2016; 30: 1079-1085.
- Yang H, Zhao H, Gao X, Huang X, Cao X, et al. Endocrine evaluation in POEMS syndrome: a cohort study. Front Endocrinol (Lausanne). 2020; 11: 536241.
- Allam JS, Kennedy CC, Aksamit TR, Dispenzieri A, et al. Pulmonary manifestations in patients with POEMS syndrome: a retrospective review of 137 patients. Chest. 2008; 133: 969-974.
- Dispenzieri A. How I treat POEMS syndrome. Blood. 2012; 119: 5650-5658.
- Humeniuk MS, Gertz MA, Lacy MQ, Kyle RA, Witzig TE, et al. Outcomes patients with POEMS syndrome treated initially with radiation. Blood. 2013; 122: 68-73.
- D'Souza A, Lacy M, Gertz M, Kumar S, Buadi F, et al. Longterm outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood 2012; 120: 56.
- Tayyaba A, Quazilbash MH. POEMS syndrome. A multisystem clonal disorder. Eur J Haematol. 2021; 106: 14-18.
- Kansagra A, Dispenzieri A, Fraser R, Estrada-Merly N, Sidana S, et al. Outcomes after autologous hematopoietic cell transplantation in POEMS syndrome and comparison with multiple myeloma. Blood Adv 2022; 6: 3991- 3995.
- Cook G, Iacobelli S, van Biezen A, Ziagkos D, LeBlond V, et al. High-dose therapy and autologous stem cell transplantation in patients with POEMS syndrome: a retrospective study of the Plasma Cell Disorder sub-committee of the Chronic Malignancy Working Party of the European Society for Blood & Marrow Transplantation. Haematologica. 2017; 102: 160.
- Tomkins O, Keddie S, Lunn MP, D'Sa S. High-dose therapy and autologous transplantation for POEMS Syndrome: effective, but how to optimise? Br J Haematol. 2019; 186: e178-e181.
- Jaccard A, Royer B, Bordessoule D, Brouet JC, Fermand JP. High-dose therapy and autologous blood stem cell transplantation in POEMS syndrome. Blood. 2002; 99: 3057.
- Kuwabara S, Misawa S, Kanai K, Kikkawa Y, Nishimura M, et al. Autologous peripheral blood stem cell transplantation for POEMS syndrome. Neurology. 2006; 66:105.
- Jurczyszyn A, Castillo JJ, Olszewska-Szopa M, Kumar L, Thibaud S, et al. (2022). POEMS Syndrome: Real World Experience in Diagnosis and Systemic Therapy-108 Patients Multicenter Analysis. Clinical Lymphoma Myeloma and Leukemia. 2022; 22: 297-304.
- Kourelis TV, Buadi FK, Kumar SK, Gertz MA, Lacy MQ, et al. Long-term outcome of patients with POEMS syndrome: an update of the Mayo Clinic experience. Am J Hematol. 2016; 91: 585-589.
- Zhao H, Huang XF, Gao XM, Cai H, Zhang L, et al. What is the best first-line treatment for POEMS syndrome: autologous transplantation, melphalan and dexamethasone, or lenalidomide and dexamethasone? Leukemia. 2019; 33: 1023-1029.
- Shibamiya A, Ohwada C, Ishii A, Mishina T, Nagai Y, et al. Successful second autologous stem-cell transplantation for patients with relapsed and refractory POEMS syndrome. Bone Marrow Transplantation, 2021; 56: 517-520.
- Autore F, Innocenti I, Sora F, Chiusolo P, Piccirillo N, et al. Successful Second Autologous Stem Cell Transplantation for Late Disease Progression after the First Procedure in POEMS Syndrome. Applied Sciences, 2022; 12: 2577.
- Yu YY, Gao XM, Zhao H, Cai H, Feng J, et al. Treatment and outcomes of POEMS syndrome: changes in the past 20 years. Blood Cancer J. 2021; 11: 1-5.
- Li J, Zhang W, Jiao L, Duan MH, Guan H-Z, et al. Combination of melphalan and dexamethasone for patients with newly diagnosed POEMS syndrome. Blood. 2011; 117: 6445-6449.
- Li J, Huang XF, Cai QQ, Wang C, Cai H, et al. A prospective phase II study of low dose lenalidomide plus dexamethasone in patients with newly diagnosed polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome. Am J Hematol. 2018; 93: 803-809.
- Nozza A, Terenghi F, Gallia F, Adami F, Briani C, et al. Lenalidomide and dexamethasone in patients with POEMS syndrome: results of a prospective, open-label trial. Br J Haematol. 2017; 179: 748-755.
- Royer B, Merlusca L, Abraham J, Musset L, Haroche J, et al. Efficacy of lenalidomide in POEMS syndrome: a retrospective study of 20 patients. Am J Hematol. 2013; 88: 207-212.
- Gao XM, Yu YY, Zhao H, Cai H, Zhang L, et al. Bortezomib plus dexamethasone as first-line therapy for patients with POEMS syndrome. Annals of Hematology. 2021; 100: 2755-2761.
- He H, Fu W, Du J, Jiang H, Hou J. Successful treatment of newly diagnosed POEMS syndrome with reduced-dose bortezomib based regimen. Br J Haematol. 2018; 181: 126-128.
- Mauermann M, Litchy W, Dyck P, Singer W, Davies J, et al. Ixazomib, Lenalidomide, and Dexamethasone (Ixa-Len-Dex) for Patients with POEMS Syndrome. Neurology. 2020; 94: 4367.
- Khan M, Stone K, van Rhee F. Daratumumab for POEMS Syndrome. Mayo Clin Proc. 2018; 93: 542-544.
- Leung B, Nelson T, Burford M. Daratumumab as First Line Therapy in a POEMS Syndrome Patient Demonstrating Atypical Features. Neurology. 2020; 94: 451.
- Gavriatopoulou M, Ntanasis-Stathopoulos I, Fotiou D, Migkou M, Eleutherakis- Papaiakovou E, et al. Upfront Daratumumab With Lenalidomide and Dexamethasone for POEMS Syndrome. Hema Sphere. 2020; 4: 3:(e381).
- Tiew HW, Sampath VS, Gallardo CA, Christopher D, Chan SSW, et al. Singleagent daratumumab for refractory POEMS syndrome. American journal of hematology. 2022; 97: E189-E191.
- Khwaja J, Keh R, Smyth D, Lunn MP, D'Sa S, Sive J. Daratumumabbortezomib- dexamethasone use in relapsed POEMS syndrome. EJHaem. 2022; 3:1021-1024.
- Xu J, Wang Q, Xu H, Gu C, Jiang L, et al. Anti-BCMA CAR-T cells for treatment of plasma cell dyscrasia: case report on POEMS syndrome and multiple myeloma. J Hematol Oncol. 2018; 11:128.
- Sekiguchi Y, Misawa S, Shibuya K, Nasu S, Mitsuma S, et al. Ambiguous effects of anti-VEGF monoclonal antibody (bevacizumab) for POEMS syndrome. J Neurol Neurosurg Psychiatry. 2013; 84: 1346-1388.
- Buxhofer-Ausch V, Gisslinger B, Stangl G, Rauschka H, Gisslinger H. Successful treatment sequence incorporating bevacizumab for therapy of polyneuropathy in two patients with POEMS syndrome. Leuk Res. 2012; 36: e98-e100.
- Badros A, Porter N, Zimrin A. Bevacizumab therapy for POEMS syndrome. Blood. 2005; 106: 1135.
- Kanai K, Kuwabara S, Misawa S, Hattori T. Failure of treatment with anti- VEGF monoclonal antibody for long-standing POEMS syndrome. Intern Med. 2007; 46: 311-313.
- Samaras P, Bauer S, Stenner-Liewen F, Steiner R, Zweifel M, et al. Treatment of POEMS syndrome with bevacizumab. Haematologica. 2007; 92:1438-1439.
- Wang C, Su W, Zhang W, Di Q, Duan M-H, et al. Serum immunoglobulin free light chain and heavy/light chain measurements in POEMS syndrome. Ann Hematol. 2014; 93: 1201-1206.
- Wang C, Huang XF, Cai QQ, Cao XX, Duan MH, et al. Prognostic study for overall survival in patients with newly diagnosed POEMS syndrome. Leukemia. 2017; 31: 100-106.
- Fu FW, Rao J, Zheng YY, Wang H-L, Yang J-G, et al. Ischemic stroke in patients with POEMS syndrome: a case report and comprehensive analysis of literature. Oncotarget. 2017: 8: 89406-89424.
- Yang H, Zhao H, Gao X, Huang X, Cao X, et al. Endocrine Evaluation in POEMS Syndrome: A Cohort Study. Front Endocrinol (Lausanne). 2020; 11: 536241.