
Case Report
Austin J Anat. 2022; 9(1): 1109.
A Case of Spinal Cord Injury Caused By Electrical Stimulation of Thoracic Spinal Cord for Treatment of Diabetic Foot
Zhou PB and Bao M*
Department of Neurosurgery, Shengjing Hospital of China Medical, University, Shenyang, China
*Corresponding author: Min Bao Department of Neurosurgery, Shengjing Hospital of China Medical University, No. 36, Sao Hao Street, Shenyang, Liaoning, 110004, China
Received: November 14, 2022; Accepted: December 17, 2022; Published: December 23, 2022
Dear Editor
The thoracic Spinal Cord Stimulator (SCS) has been widely accepted as a treatment option for diabetic foot and is gaining acceptance. This case highlights a previously unreported potential complication of the SCS. A 66-year-old man had long-standing diabetes (30 years) and hypertension (10 years), accompanied by diabetic foot and generalized psoriasis. The patient reported pain in the bilateral lower extremities, especially in both feet, which was significant at night and when walking. His Visual Analog Scale (VAS) pain score was 10/10. The pain was accompanied by slight numbness without sensory disturbance. These symptoms gradually exacerbated over 2 months. His Quality of Life Scale (QOLS) score was 186, and the skin temperature of both his feet decreased at rest. Following conservative treatment elsewhere, the abovementioned symptoms remained refractory; the patient presented to our hospital for further treatment. SCS was implanted on June 6, 2021 for pain relief.
After admission, we completed preoperative examinations and scheduled surgery under C-arm guidance. Because of severe psoriasis on the skin surface of T11 and T12, the possibility of impaired incision healing was considered. The skin over T9 and T10 was incised after careful consideration (Figure 1). Conventional upward insertion of the electrode into the epidural space of T11 and T12 vertebral bodies was abandoned in favor of downward insertion of the electrode into the epidural space of T9 and T10 vertebral bodies (Figure 1); the same treatment outcome was eventually achieved. An electrode (model: 565 DEFINE 2 * 8; Medtronic, USA) was implanted, and the resistance was normal intraoperatively. A conventional initial voltage of 0.5 V, pulse width of 210μs, and frequency of 40 Hz were adopted for testing. The patient stated that the sensation of electricity passing through the lower limbs was significant and involved full coverage of both feet. We connected the SCS to a temporary, external stimulator. Once safely returned to ward, we conducted postoperative adjustment and set the stimulat or with the following parameters: voltage, 0.1 V; pulse width, 210μs, and frequency, 40 Hz. When the voltage was modulated to 0.5 V, the patient’s sensation of current stimulation in the lower limbs was un ideal; thus, we gradually increased the voltage to 1.5 V. At this point, the lower limbs could not be autonomously controlled, the feet were unresponsive to external stimulation, and with T12 as the transverse section, all spinal cord reflexes below disappeared, specifically manifesting as flaccid paralysis, reduced muscle tension, disappearance of knee and tendon reflexes, and failure to elicit pathological reflexes. Fecal and urinary incontinence was absent, consistent with “acutephase spinal cord shock.” The thoracic vertebra was examined by plain radiography after considering ongoing methylprednisolone for Spinal Cord Injury (SCI). After eliminating fractures or acute lesions, we switched off the external temporary stimulator, and the motor and sensory abnormalities of the lower limbs disappeared. On postoperative day 1, we attempted re-initiate the stimulator. When the voltage was adjusted to 1.15 V, the patient’s sensation of current in the lower limbs was satisfactory, and no abnormalities were found. The temperature of the patient’s feet was significantly higher postoperatively than in the preoperative period (Figure 1); the pain in the lower limbs resolved. The VAS pain score was 0/10, postoperatively. The patient’s condition remained stable throughout his hospital stay. Before discharge, his pain had significantly improved, with normal motor and sensory functions on both lower limbs. He returned to the hospital 2 weeks after discharge and was treated with an implantable pulse generator. During the 3-month follow-up period, the patient recovered well, and the QOLS score was 253.
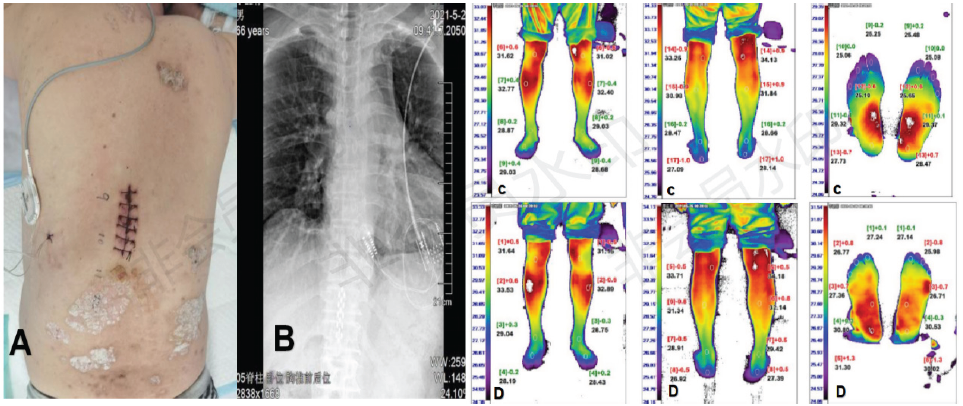
Figure 1: (A) Because of psoriasis on the skin surface at the T11 and T12 levels, the T9 and T10 levels were selected for incisions.
(B) The electrode is fixed in the epidural space of the T11 level. (C) Preoperative infrared thermography showing the
skin temperature of each part of both the lower limbs. (D) Postoperative infrared thermography showing the skin temperature
of each part of both the lower limbs.
Discussion
It has been reported that 10,000–12,000 traumatic SCI cases occur each year. Generally, the common mechanism of these injuries is vehicular collision or expressway accidents. We present a unique case wherein SCI was secondary to the implantation of a thoracic SCS. The treatment of diabetic foot with a thoracic SCS has been widely accepted and is being increasingly applied. During the postoperative period, this patient’s injury and recovery were more consistent with spinal cord concussion, which highlights a (previously unreported) potential complication of SCS and reveals the electrophysiological characteristics of spinal cord concussion.
Spinal cord stimulation is a neuromodulation technique for relieving chronic pain. The most common indication of spinal cord stimulation treatment is post-laminectomy syndrome, and sometimes, neuropathic pain will occur after lumbar spine surgery [1]. The efficacy of spinal cord stimulation for diabetic foot is noteworthy. Presumably, the electrical pulse activates the Aβ fibers in the dorsal column, changes the thresholds of sensation and pain, and modifies advanced cortical processing [2]. Meanwhile, spinal cord stimulation inhibits sympathetic vasoconstriction and stimulates parasympathetic vasodilation, thus alleviating lower limb ischemia and salvaging the limb.
However, the complications caused by postoperative program control have rarely been investigated previously. Improper program control may reduce the efficacy of the SCS system in relieving pain, which in turn exacerbates the harmful effects of stimulation, leading to tissue burns or electric shocks [3]. The process of postoperative program control, with an increase in voltage, can be divided into “perception domain,” “treatment window,” and “discomfort domain,” which may vary per patient. People have different threshold heights and widths. The general concept of perception domain is that there is a sensation of current in the region of the hips or legs of the patient, although it does not reach the feet; the treatment window is also known as the comfort zone, i.e., the current covers the whole feet, achieving the purpose of the treatment and not causing any discomfort to the patient; the discomfort domain is often intolerable, and normal life is affected by excessive current. To the best of our knowledge, there is no report of SCI caused by an SCS. Similarly, this case should raise physicians’ awareness of risks involved in inserting a space-occupying block in the epidural space. The space in the spinal canal in the cervical and thoracic regions is smaller, requiring special attention [4]. Most patients whose neck and upper limb pain improve with medical treatment have concurrent conditions, such as degenerative disc disease. In this population, spinal stenosis may also exist to an extent, and the risk of injury increases among patients who use an SCS. The patient in this report benefited from the SCS. With patients increasingly opting for SCS implantation, potential long-term risks must be considered.
Acknowledgements
MB and PBZ participated in patient management; PBZ collected and analyzed patient data; MB provided technical support; PBZ wrote the manuscript; MB was responsible for critical revisions of the manuscript.
Funding
This study was supported by Liaoning Provincial Natural Science Grant (No. 20180530001).
Informed Consent
This study has obtained informed consent from human experiments. And always abide by the human subject's right to privacy.
References
- Baber Z, Erdek MA. Failed back surgery syndrome: current perspectives. J Pain Res. 2016; 9: 979–87.
- Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 2018; 18: 1048–67.
- Markosian C, Taruvai VS, Mammis A. Neuromodulatory hacking: a review of the technology and security risks of spinal cord stimulation. Acta Neurochir (Wien). 2020; 162: 3213–9.
- Falowski S, Ooi YC, Sabesan A, Sharan A. Spinal cord injury induced by a cervical spinal cord stimulator. Neuromodulation. 2011; 14: 34–7.