Editorial
With a steadily increasing world population, infertility and sub fertility have been distressed a significant proportion of humanity. The emotional impact of infertility has been described via several clinical observations. Up to half of all infertile couples, male infertility plays a critical role, and in some cases, the causes of infertility remain unknown. Recent progress towards understanding male infertility has been demonstrated that quality and quantity of spermatozoa are one of the leading causes of infertility. Since sperm’s ability to fertilize an oocyte is largelya molecular biochemical event; in-depth understanding of sperm biology could provide useful guidelines for clinicians and researchers to sustain patient’s hope. In this review, we discussed some basic events in spermatozoa that confer its ability for fertilization, which need to be understood in order to answer the global issue of male infertility.
Keywords: Infertility; Spermatozoa; Motility; Capacitation; Acrosome Reaction; Fertilization
Introduction
Infertility of both male and female has become a global concern [1] because approximately 15% of couples are suffering from this problem and around 50% cases male partner of a couple is responsible for not having any child. Based on the review of existing literature, Agrawal et al. [2] have reported an alarming percentage of male factors infertility across the globe (Figure 1). One of the common causes of male infertility is the abnormality of spermatozoa [1,2]. Some preliminary studies have suggested that sperm counts and motility are the major factors of male infertility [1-3]. However, results of most recent studies indicated that men with very low sperm counts and motility sometimes may have babies and vice versa [4-6]. Therefore, the ability of spermatozoa to fertilize an oocyte is mainly a biochemical event at the molecular level that needs to be understood in order to answer the global concern of male infertility.
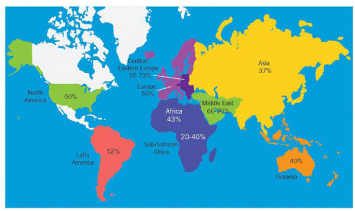
Figure 1: Illustration represents percentage of male factor infertility in North
America, Latin America, Africa, Europe, Central/Eastern Europe, Middle East,
Asia, and Oceania. The image was adapted from Agarwal and others (2015),
reviewing the male infertility around the globe. Reprod Biol Endocrinol. 13:37
[2].
Sperm Biology vs Male (In) Fertility
Formation and maturationof spermatozoa
A spermatozoon is the male sex cell produced through a unique process called spermatogenesis, which starts due to the differentiation process of spermatogonial stem cells. The entire process of sperm production includes a complex interaction of three consecutive phases of cellular proliferation and differentiation [1]. First, spermatogonial cells divided mitotically to produce an optimum number of spermatogonia that give rise to diploid primary spermatocytes. Second, the primary spermatocyte divides meiotically (meiosis I) into two secondary spermatocytes. Third, each secondary spermatocyte undergoes second meiosis (meiosis II) and divides into two round spermatids. Subsequently, the roundspermatids undergo remodeling of their nuclear, chromatin, and cellular components, finally transform to spermatozoa into the lumen of the seminiferous tubule by a process named spermiogenesis [7]. The processes of spermatogenesis have been depicted in Figure 2. Spermatozoa then undergo a maturation process and acquire functionality during their journey from proximal to the distal end of the epididymis [8]. The entire processes of spermatogenesis, as well as epididymal maturation of spermatozoa are highly sensitive to the fluctuations of the environment, particularly hormones and temperature. In addition, dietary deficiency (e.g. vitamins E, B, and A), habitat (e.g. smoking, alcohol consumption), exposure to the toxic metals (e.g. cadmium and led), radiation, pesticides, chemotherapy, and environmental contaminants (e.g. endocrine disrupting chemicals) may directly affect the processes, leading to the abnormal spermatogenesis, low sperm count, and male infertility [9-15]. Therefore, in order to maintain proper reproductive health individual should minimize/ avoid such risk factors.
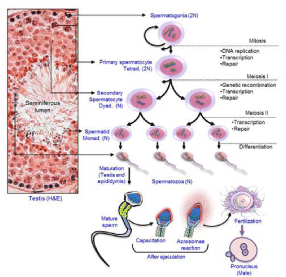
Figure 2: Illustration represents the cellular, genetic, and chromatin
modifications during spermatogenesis. Male primordial germ cells
(spermatogonia) differentiate to primary spermatocytes that subsequently
undergo genetic changes to produce round spermatids. Next, the round
spermatids participate in spermiogenesis and finally produce permatozoa.
Following ejaculation, spermatozoa must undergo capacitation and the
acrosome reaction, prerequisite for fertilization. The left panel of the figure
shows histological section of mice testis with different spermatogenic cells,
stained with Hematoxylin and Eosin staining (H&E). See also the main text
for the description of the illustration. The figure has been modified, and citing
the original source published in Int J Endocrinol, 2013 [1].
Morphological features of spermatozoa
All men produce a large proportion of morphologically (size and shape) abnormal spermatozoa. According to the new edition of the World Health Organization manual of semen analysis, only 4-15% of total spermatozoa are being considered as normal [16]. A typical mammalian spermatozoon contains an oval‒shaped head, a well‒defined acrosome (covers about 40-70% of the sperm head), and intake neck, midpiece, and tail. In the early 1900s, morphological features were considered single most important criteria to detect the fertilization capability of a spermatozoon [17]. Although this statement later has proven to be inconsistent, there is a strong positive correlation between the percentage of normal spermatozoa with fertilization rate, both in-vivo and in-vitro [16]. Now, therefore, evaluation of sperm morphology has become a routine practice in infertility clinics to examine fertility competence of a male, as well as to decide whether a couple is capable of In-Vitro Fertilization (IVF) to attempt pregnancy or not.
Sperm motility and motion kinematics
Motility is one of the important characteristics associated with the fertilizing capability of spermatozoa. Therefore, measuring the fraction of perfectly motile spermatozoa probably is one of the most straightforward approaches used worldwide to detect male fertility [18,19]. In order to make this benchmark indicator more reliable in predicting fertility, different infertility clinics optimize their own experience of the correlation between semen analysis results (motility and motion kinematics parameters) and subsequent fertility outcomes.
After production in testis, mammalian spermatozoa undergo maturation process as they travel through epididymis [20]. Subsequently, spermatozoa acquire cholesterol, protein, etc., from the epididymis, which is an essential preliminary step for gaining motility [20,21]. Although spermatozoa gain oxygen from the cauda epididymis, they remain immotile [22]. In a later stage, spermatozoa start a strong flagellar movement when they come and contact with the seminal fluid containing a high concentration of HCO3 − and Ca2 +ions [1,22]. The movement of the both ions into the spermatozoa regulates metabolism of cyclic Adenosine Monophosphate (cAMP) via triggering a unique type of Adenylyl Cyclase (sACY) located in sperm membrane (Figure 3). In addition, HCO3 − has also been associated withan increase in the intracellular pH (pHi). As a consequence of sACY activation, increased levels of intracellular cAMP activated the Protein Kinase-A (PKA). Further activation of PKA in spermatozoa is gained through phosphorylation of PKA substrates [23]. Together the increased levels of cAMP and phosphorylated PKA substrates in spermatozoa trigger the early activation of the sperm motility [1,9,24- 26] (Figure 3). Therefore, if any chemicals or environmental factors are capable of manipulation these molecular cascades in spermatozoa, it will definitely affect the sperm motility and male fertility.
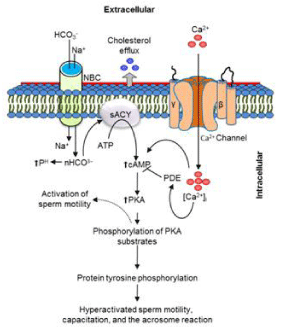
Figure 3: Illustration represents molecular changes in spermatozoa
responsible for the motility activation, capacitation, and the acrosome
reaction. The sperm motility is activated by the phosphorylation of protein
kinase-A (PKA) substrates in a media containing HCO3
−
and Ca2
+ sources. At
the molecular level, PKA is activated by Ca2
+ and HCO3
−
mediated triggering
of the transmembrane atypical adenylyl cyclase (sACY). In these cases,
Ca2
+ and HCO3
−
are transported across cell membrane via Ca2
+ channel
(Casper) and Na+/HCO3
−
cotransporter (NBC), respectively. Simultaneously,
incubation of spermatozoa either in-vivo (female reproductive tract) or in-vitro
(in a specialized media) for extended period of time increased the tyrosine
phosphorylation, responsible for the capacitation, the acrosome reaction,
and changes in the motility pattern known as hyper activation. On the other
hand, inhibition of Phosphordiesterase (PDE) has been increased cAMP
levels, subsequently affects sperm motility. See also the main text for the
description of the illustration. The figure has been modified, and cite the
original source published in Proc Natl Acad Sci U S A., 2009 [22] and Reprod
Biol Endocrinol., 2004 [38].
Recently it has been demonstrated that exposure to endocrine disrupting chemicals (e.g. bisphenol-A, sodium fluoride, benzopyrene, and so no) has been linked with the significantly decreased sperm motility [9,25,27]. The decreased motility of spermatozoa due to the exposure of the endocrine disrupting chemicals were associated with altered PKA activities and phosphorylation of sperm proteins in tyrosine residue [9,25]. The similar effects on sperm motility were also demonstrated in another study due to in-vitro exposure of toxic chemicals sodium nitroprusside [28]. In another study Yoon et al., [29,30] demonstrated that addition of cryoprotectant agent to bull spermatozoa during cryopreservation decreased the motility via alteration of mitochondrial activities in spermatozoa. Therefore, future researches should focus on identifying other agents; especially environmental factors that are capable of modifying male fertility via regulation of the sperm motility.
Capacitation and the acrosome reaction
Ejaculated mammalian spermatozoa are unable to fertilize an oocyte even they are mature or morphologically normal [18,31,32]. Therefore, spermatozoa must undergo a chain of biochemical and physiological modifications that enable its bindings and penetration into an oocyte [33]. These essential modifications in mammalian spermatozoa responsible for fertilization, commonly termed as capacitation [34-36]. The acrosome reaction is another necessary event in spermatozoa and also similarly important for sperm‒ oocyte fusion [33]. Jin et al., [37] reported that fertilizing murine spermatozoa start their acrosome reaction before contact with the oocyte zona pellucida. In fact, clinical uses of IVF only become possible by the discovery of capacitation as well as the acrosome reaction. Both events are regulated by a complex interactions of several cascades, such as an influx of intracellular calcium, availability of bicarbonate ions, changes of sperm membrane fluidity, cAMP and PKA activity, and protein tyrosine phosphorylation in spermatozoa [1,18,19,22,24,38] (Figure 3). Although these changes in spermatozoa during capacitation/the acrosome reaction occur in the female genital tract in vivo; however, it can also be achieved in-vitro in a specialized media containing an appropriate concentration of bicarbonate, calcium, and serum albumin [38]. Similar to the early activation of the sperm motility, increased levels of pHi due to transmembrane movement of the HCO3 − also has been noticed during these changes. Salicioni et al. [23] reported that serum albumin that used to induce capacitation in vitro is responsible for depletion of plasma membrane cholesterol in spermatozoa, which subsequently has given raise the other capacitation conferring cholesterol-binding compounds named cyclodextrins. Tyrosine phosphorylation, on the other hand, another most important event associated with capacitation and the acrosome reaction (Figure 3).
Recently from our laboratory we were able to identify several chemicals/ toxic chemicals (e.g. sodium nitroprusside; CK-636, nutlin-3a, 4,4’-diisothiocyanostilbene-2,2’-disulfonic acid, and deamino vasopressin) and environmental contaminants (especially endocrine disrupting chemicals, such as bisphenol-a, sodium fluoride,genistein, 4-tert-octylphenol, and benzopyrene) that potentially regulate tyrosine phosphorylation in spermatozoa through a PKA-dependent/independent mechanism, simultaneously affect the capacitation/the acrosome reaction and succeeding fertility of mouse spermatozoa [9,25-28,39-42]. Therefore, basic knowledge of sperm capacitation may increase our understanding to unravel the mystery of male infertility. Recently, Kwon et al. [43] reported that evolution of boar sperm capacitation status showed significant correlation with subsequent fertility, while sperm motility and motion kinematics parameters represented a statistically non-significant correlation with litter size. Therefore, evolution of capacitation status of spermatozoa together with conventional semen analysis might consider in order optimizing the fertility prediction in human and domestic animal species. In contrast, other studies have reported that premature acrosome reaction may result in altered mitochondrial function and chromatin decondensation of spermatozoa, which have potential harmful effects on sperm viability and fertility [44,45]. Usually, a component of egg zona pellucida induces the acrosome reaction in spermatozoain situ, following binding. Therefore, if the acrosome reaction has taken place early prior to the spermatozoa reaching to the oocyte is incapable to penetrate the zona pellucida [46,47]. Therefore, detection of premature acrosome reaction/ sperm acrosomal status could be one of the very useful methods for the evaluation of sperm fertility [48,49].
Conclusion
In conclusion, this review will open new windows to investigate male infertility. Firstly, it identifies several molecular mechanisms that regulate sperm’s ability to function normally. Secondly, it serves as a proof of principle to optimize male (in) fertility prediction. Since, this manuscript described several findings mainly derived from in-vitro studies to correlate sperm function and male fertility, therefore future research should be targeted using in-vivo model and to prove whether these correlations is also existed in-vivo. On the other hand, some other factors such as genetic and epigenetic factors are also very important in order to optimize male fertility. There is increasing recognition of the contribution of both factors to the causation of male infertility. Therefore, studies are also required to search relationships between genetic and epigenetic alteration in spermatozoa and associated male infertility.
References
- Rahman MS, Lee JS, Kwon WS, Pang MG. Sperm proteomics: road to male fertility and contraception. Int J Endocrinol. 2013; 2013: 360-986.
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015; 13: 37.
- Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm's journey to and interaction with the oocyte. J Clin Invest. 2010; 120: 984-994.
- Antinori M, Licata E, Dani G, Cerusico F, Versaci C, d'Angelo D, et al. Intracytoplasmic morphologically selected sperm injection: a prospective randomized trial. Reprod Biomed Online. 2008; 16: 835-841.
- Gosálvez J, Migueles B, López-Fernández C, Sanchéz-Martín F, Sáchez-Martín P. Single sperm selection and DNA fragmentation analysis: the case of MSOME/IMSI. Nat Sci. 2013; 5: 7-14.
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015; 104: 1398-1405.
- O'Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2015; 4: 623-979.
- Dacheux JL, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction. 2013; 147: 27-42.
- Rahman MS, Kwon WS, Lee JS, Yoon SJ, Ryu BY, Pang MG. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci Rep. 2015; 5: 9169.
- Watanabe T, Ohkawa K, Kasai S, Ebara S, Nakano Y, Watanabe Y. The effects of dietary vitamin B12 deficiency on sperm maturation in developing and growing male rats. Congenit Anom (Kyoto). 2003; 43: 57-64.
- Gaur DS, Talekar MS, Pathak VP. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010; 53: 35-40.
- Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect. 2008; 116: 1473-1479.
- Gorpinchenko I, Nikitin O, Banyra O, Shulyak A. The influence of direct mobile phone radiation on sperm quality. Cent European J Urol. 2014; 67: 65-71.
- Bretveld R, Brouwers M, Ebisch I, Roeleveld N. Influence of pesticides on male fertility. Scand J Work Environ Health. 2007; 33: 13-28.
- Chan PT . Fertility after cancer in men. Can Urol Assoc J. 2009; 3: 223-224.
- Menkveld R. Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J Androl. 2010; 12: 47-58.
- Moench GL, Holt H. Sperm morphology in relation to fertility. Am J Obstet Gynecol. 1931; 22: 199-210.
- Kwon WS, Rahman MS, Lee JS, Kim J, Yoon SJ, Park YJ, et al. A comprehensive proteomic approach to identifying capacitation related proteins in boar spermatozoa. BMC Genomics. 2014; 15: 897.
- Kwon WS, Rahman MS, Lee JS, Yoon SJ, Park YJ, Pang MG. Discovery of predictive biomarkers for litter size in boar spermatozoa. Mol Cell Proteomics. 2015; 14: 1230-1240.
- Cornwall GA, van Horsten HH. Sperm maturation in the epididymis: Role of segment-specific microenvironments. In: Carrell DT, editor. The genetics of male infertility. Totowa, N.J. Humana Press. 2007; 211-231.
- Fraser LR. New insights into possible causes of male infertility. Hum Reprod. 1999; 14 Suppl 1: 38-46.
- Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci USA. 2009; 106: 667-668.
- Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, et al . Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl. 2007; 65: 245-259.
- Rahman MS, Kwon WS, Pang MG. Calcium influx and male fertility in the context of the sperm proteome: an update. Biomed Res Int. 2014; 2014: 615-841.
- Kim J, Kwon WS, Rahman MS, Lee JS, Yoon SJ, Park YJ, et al . Effect of sodium fluoride on male mouse fertility. Andrology. 2015; 3: 544-551.
- Lee JS, Kwon WS, Rahman MS, Yoon SJ, Park YJ, Pang MG. Actin-related protein 2/3 complex-based actin polymerization is critical for male fertility. Andrology. 2015; 3: 937-946.
- Mohamed el-S, Song WH, Oh SA, Park YJ, You YA, Lee S, et al . The transgenerational impact of benzo(a)pyrene on murine male fertility. Hum Reprod. 2010; 25: 2427-2433.
- Rahman MS, Kwon WS, Lee JS, Kim J, Yoon SJ, Park YJ, et al. Sodium nitroprusside suppresses male fertility in vitro. Andrology. 2014; 2: 899-909.
- Yoon SJ, Kwon WS, Rahman MS, Lee JS, Pang MG . A novel approach to identifying physical markers of cryo-damage in bull spermatozoa. PLoS One. 2015; 10: 0126232.
- Yoon SJ, Rahman MS, Kwon WS, Park YJ, Pang MG . Addition of Cryoprotectant Significantly Alters the Epididymal Sperm Proteome. PLoS One. 2016; 11: 0152690.
- Kwon WS, Rahman MS, Pang MG . Diagnosis and prognosis of male infertility in mammal: the focusing of tyrosine phosphorylation and phosphotyrosine proteins. J Proteome Res. 2014; 13: 4505-4517.
- Kwon WS, Rahman MS, Ryu DY, Park YJ, Pang MG. Increased male fertility using fertility-related biomarkers. Sci Rep. 2015; 5: 15654.
- Yanagimachi R. Mammalian Fertilization. In: Knobil ENJ, editor. Physiology of Reproduction. New York, Raven Press. 1994; 189-317.
- CHANG MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951; 168: 697-698.
- Ickowicz D, Finkelstein M, Breitbart H. Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl. 2012; 14: 816-821.
- Zaneveld LJ, De Jonge CJ, Anderson RA, Mack SR . Human sperm capacitation and the acrosome reaction. Hum Reprod. 1991; 6: 1265-1274.
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA. 2011; 108: 4892-4896.
- Naz RK, Rajesh PB . Role of tyrosine phosphorylation in sperm capacitation / acrosome reaction. Reprod Biol Endocrinol. 2004; 2: 75.
- Kwon WS, Park YJ, Kim YH, You YA, Kim IC, Pang MG. Vasopressin effectively suppresses male fertility. PLoS One. 2013; 8: 54192.
- Kwon WS, Park YJ, Mohamed el-SA, Pang MG . Voltage-dependent anion channels are a key factor of male fertility. Fertil Steril. 2013; 99: 354-361.
- Ryu DY, Kim YJ1, Lee JS, Rahman MS, Kwon WS, Yoon SJ, et al. Capacitation and acrosome reaction differences of bovine, mouse and porcine spermatozoa in responsiveness to estrogenic compounds. J Anim Sci Technol. 2014; 56: 26.
- Shukla KK, Kwon WS, Rahman MS, Park YJ, You YA, Pang MG. Nutlin-3a decreases male fertility via UQCRC2. PLoS One. 2013; 8: 76959.
- Kwon WS, Rahman MS, Lee JS, You YA, Pang MG. Improving litter size by boar spermatozoa: application of combined H33258/CTC staining in field trial with artificial insemination. Andrology. 2015; 3: 552-557.
- Chaveiro A, Machado L, Frijters A, Engel B, Woelders H . Improvement of parameters of freezing medium and freezing protocol for bull sperm using two osmotic supports. Theriogenology. 2006; 65: 1875-1890.
- Wongtawan T, Saravia F, Wallgren M, Caballero I, Rodríguez-Martínez H. Fertility after deep intra-uterine artificial insemination of concentrated low-volume boar semen doses. Theriogenology. 2006; 65: 773-787.
- Florman HM, Ducibella T. Fertilization in mammals. In: Knobil and Neill's Physiology of Reproduction, 3rd edn, Elsevier Academic Press, St. Louis, MO. 2006; 55-112.
- Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R . Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci U S A. 2011; 108: 20008-20011.
- Sutovsky P, Kennedy CE. Biomarker-based nanotechnology for the improvement of reproductive performance in beef and dairy cattle. Industrial Biotechnology. 2013; 9: 24-30.
- Odhiambo JF, Sutovsky M, DeJarnette JM, Marshall C, Sutovsky P. Adaptation of ubiquitin-PNA based sperm quality assay for semen evaluation by a conventional flow cytometer and a dedicated platform for flow cytometric semen analysis. Theriogenology. 2011; 76: 1168-1176.
