Abstract
A spinal pattern generator controls ejaculation in male rats. Spinal pattern generators may display oscillatory bursting activity that shape the rhythmic motor patterns as a result of the intrinsic or conditioned oscillatory properties of its neuronal elements. The spinal pattern generator for ejaculation could exhibit oscillatory activity. The aim of the present study was to show the oscillatory activity of the spinal pattern generator for ejaculation in male rats. To this aim, we analysed the ejaculatory responses of spinal male Wistar rats of different ages, different ejaculatory conditions, subjected to different physiological or pharmacological stimuli within the frame of the theory of oscillatory behaviour. State variables, phases and periods of electromyography recordings of the genital motor patterns of ejaculation were obtained from the bulbospongiosus muscles. Recordings of the spontaneous and sensorially-induced genital motor patterns of ejaculation, obtained under the different physiological conditions or pharmacological treatments, revealed the oscillatory behaviour of the spinal generator for ejaculation which showed striking variations among rats of different ages, ejaculatory conditions and subjected to distinct stimuli, including an intense sexual training scheme. In conclusion, the spinal pattern generator for ejaculation behaves as a neural oscillator whose function can be modified by internal and external demands. The variability in the oscillatory activity of the ejaculation generator is a prominent feature that should be taken into consideration when characterising the most common ejaculatory dysfunctions.
Keywords: Ejaculation Oscillator; Spinal Oscillator; Spinal Pattern Generator; Spinal Cord; Ejaculation; Ejaculatory Dysfunction
Introduction
Rhythmic motor behaviours such as respiration, locomotion, chewing and ejaculation are controlled by neuronal networks known as central pattern generators (CPG) [1-3]. CPGs are constituted by specialised groups of neurones that act as local control circuits and neural command centres capable of generating organised rhythmic patterns of motor activity [4-9]. CPGs can function in the absence of higher centres influence and sensory feedback and can be activated in a spontaneous manner maintaining a proper rhythm [10,11]. Two major conceptual models have been proposed to account for the source of rhythmicity in CPGs: the pacemaker theory, which suggests the existence of inherent membrane potential oscillations in some neurones of the network that act as leaders in synchronising firing activities of non-pacemaker interneurones and the emergent network theory, in which the rhythm arises from a pool of synaptically interacting excitatory interneurones that synchronize their spiking activities [12]. It has been proposed that the CPG pacemaker neurones serve as the source of the rhythm and their synaptic interactions with other network elements can modify this rhythm, but the inherent activity of the pacemaker neurons to promote rhythmic activity remains dominant [13]. Pacemaker neurones exhibit oscillations that result from the combination of their intrinsic electric properties and their synaptic interactions [14]. In neuronal circuits with excitatory activity at high- or slow-frequency, oscillations occur to finely shape and time the rhythmic motor patterns [14,15]. A tonic excitatory drive is widely accepted to be necessary to generate specific rhythms in CPG networks [12,16] with glutamate playing an essential role, as the main excitatory neurotransmitter, through the activation of N-methyl-D-aspartate (NMDA) receptors located at the CPG [12,16]. However, activation of NMDA receptors alone is not sufficient to lead pacemaker neurones to oscillate and other mechanisms, such as those elicited by afferent inputs, are required to generate neural oscillations [15]. Although in some rhythmic behaviours such as ejaculation [17,18], it has been demonstrated that the rhythmic motor response appears in the electromyogram almost immediately after electrical micro stimulation is applied to CPG neurones, it is not known whether oscillations directly give rise to rhythmic neuronal firing. The doubt arises because the electrically-induced CPGs oscillation frequencies are often lower than those seen in natural or fictive motor responses [16-18]. Nevertheless, the oscillatory properties of neurones of the CPGs might help to shape and time their rhythmic firing rather than to generate the locomotion rhythm [19]. Thus, oscillatory properties of CPGs can control and regulate the locomotor rhythmicity [12] and cycle duration [20] and consolidate the robustness of the rhythmic motor output [12].
In urethane anaesthetised and spinalised rats with intact afferent pathways, the mechanical sensory stimulation applied to genitalia evokes a highly rhythmic motor pattern, synchronically registered in all striated muscles associated with the genital tract [21]. This motor pattern is termed the genital motor pattern of ejaculation (GMPE) and is comprised of two clear components: a first ejaculatory motor train and an after discharge component (Figure 1) [21]. The GMPE is under the control of the spinal generator of ejaculation (SGE) and it drives the rhythmic expulsion of seminal secretions, supports the concomitant mechanical expression of phasic penile erections and promotes changes into the intraurethral pressure [3 and 17, for review]. Although the precise structure, location and function of the spinal generator for ejaculation is poorly understood, several studies suggest that it could be structured by pacemaker cells, [3] located at the lamina X of the spinal cord [21-24], which in addition could exhibit oscillatory activity.
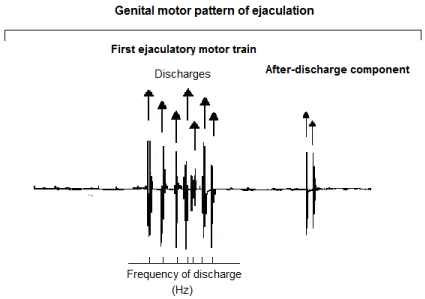
Figure 1: Polygraphic tracing showing a typical genital motor pattern of
ejaculation (GMPE) in the spinal male rat. The GMPE is composed by two
main components: a first ejaculatory motor train and an after-discharge
component. For the analysis of the EMG rhythmic motor pattern arrows
indicate individual muscular contractions and bottom trace indicates the
number of motor discharges during 10 seconds of recording (Frequency of
discharge). The after-discharge component of the GMPE was not considered
for its EMG analysis.
Thus, it has been shown that removal of descending inhibitory pathways and genital afferents that project to this spinal generator for ejaculation [21,25,26] uncovers an intraspinal rhythm of activation of the GMPE [21,23] that occurs at intervals of 3.5 min [21]. Experimental manipulations carried out to force the spinal generator for ejaculation to discharge at different frequencies in response to sustained sensory inputs, though facilitating the GMPEs, do not alter the intraspinal rhythm of activation nor the expression of the GMPE components [21], suggesting that the ejaculatory rhythm is generated by the spontaneous bursting activity of pacemaker neurones. Moreover, GMPEs are expressed after activating glutamatergic NMDA receptors located at the SGE cells, and this mechanism has been considered to be a key signal for the activation of the spinal generator of ejaculation [27]. Finally, electrical micro stimulation of neurones of the SGE initiates and maintains the GMPE [17,18].
Analysis of the functioning of the generator for ejaculation showed the concomitant facilitation and inhibition of the bursting activity in genital muscles during its activation [28], a different ejaculatory bursting activity in neonatal and in adult animals [29], as well as among sluggish, normal, or rapid ejaculating adult male rats [18]. Data have also shown that the intraspinal rhythm of activation of the ejaculation generator can be interrupted or significantly modified after the systemic administration of drugs with excitatory or inhibitory actions [30-32] and by repeated sensory stimulation [21- 23]. Altogether, these data also suggest that when activated, the SGE displays oscillatory activity that might be driving divergent modes of oscillation shaping the GMPE.
The variables of an oscillator can be described mathematically as state variables or parameters and oscillator periods and its phases [33]. The value of the state variables is affected by the oscillator itself and when the oscillator is held constant, these variables determine the future course of the dynamic system. An oscillatory period is the time required for a round-trip cycle of events, and the phases of an oscillator are fractions of that period [33]. The spinal generator for ejaculation exhibits some variations in its state variables, phases and periods that can be useful for the analysis of its potential oscillatory properties. Relevant information regarding the oscillatory activity of the generator for ejaculation could explain the biological variations in the ejaculatory response seen in different populations of ejaculators. In addition, knowledge on the features of the oscillatory activity of the generator for ejaculation could lead to the establishment of physiological or pharmacological strategies to regulate its functioning. In this study we hypothesized that if the spinal ejaculation generator exhibits oscillatory activity it would be influenced by different stimuli and modulated by intrinsic spinal mechanisms, but the basic rhythm pattern would remain unchanged. The aim of this study was to characterize the oscillatory activity of the ejaculation generator by evaluating the expression of the GMPE: a) in response to sensory stimulation, b) as a consequence of stimulatory and inhibitory pharmacological agents administered acutely or chronically, c) in animals of different ages, d) in rats of the different ejaculatory populations, and e) in response to repeated intense sexual training.
Materials and Methods
Animals
Sexually experienced male Wistar rats of different ages were used. Animals were housed by groups (five rats per cage) under an inverted LD cycle 12:12 h, at 22ºC, and with free access to food and water. The Local Committee of Ethics on Animal Experimentation approved all experimental procedures, which followed the regulations established in the Mexican official norm for the use and care of laboratory animals “NOM-062-ZOO-1999”.
Sexual behaviour observations
Previous to their assignment to a given ejaculatory category, male rats received five sexual behaviour tests with receptive females. Female receptivity was induced by the sequential subcutaneous administration of oestradiol valerianate (4μg/rat, s.c.). Followed 44 hours later by progesterone (2mg/animal, s.c.). Behavioural observations were conducted 4 hours after progesterone administration and 2 hours after the onset of darkness. Males were introduced into a cylindrical observation cage and a 5 min adaptation period was allowed. Thereafter a receptive stimulus female was introduced and sexual behaviour was recorded during 45 min. The sexual behaviour parameters monitored were: intromission latency – time from introduction of the female until the first mount with pelvic thrusting and vaginal penetration (intromission), and ejaculation latency – time from the first intromission until ejaculation. Among the sexually active males, the ejaculation latencies of the first ejaculatory series recorded in the last three sessions were used to classify them as male rats with rapid ejaculation latency (when shorter than 10 min in average), with intermediate ejaculation latency (when ranging from 15 to 20 min) and rats with retarded ejaculation latency (when longer than 30 min in average).
General surgical procedures
All animals were urethane-anaesthetised (0.7g/kg. i.p.) assessing the adequacy of anesthesia by the absence of a withdrawal reflex after noxious paw pinch. The bulbospongiosus genital muscles were identified after a surgical incision on the perineum and two platinum wires (Grass) were inserted into the muscles to record electromyographic (EMG) activity, which was registered on a polygraph (Grass M7). For a better visualisation of the genital activity associated to the rhythmic motor pattern, an additional surgery was performed to expose the bulbar portion of the penis and its anatomical connections with the striated bulbospongiosus muscles. The right femoral vein was cannulated for drug administration. At the end of the surgery the spinal cord was blunt transected at T6 spinal cord level.
Activation and recording of the rhythmic genital motor pattern of ejaculation
Immediately after spinal cord transection, the GMPE is spontaneously expressed with a mean latency of 1 to 3 min. In order to assess the ability of the spinal cord to produce the GMPE response and for comparison purposes, after spinalisation, one spontaneous GMPE was allowed to express and it was recorded in the bulbospongiosus muscles. This muscle was selected as a monitor of the genital muscles activity since it discharges during ejaculation in synchrony with all genital muscles, and given its superficial position in the perineum. The GMPE was evoked by mechanical stimulation of the urethra, which consisted in its distension produced by the injection of saline solution with a syringe pump (200 μl/min), during 10 seconds, through a PE-50 catheter (0.965mm OD) inserted into the pelvic urethra via a bladder incision, while occluding the penis meatus to achieve an intraurethral pressure that ranged from 20-30 mm Hg. The GMPE was repeatedly activated by urethral stimulation until its inhibition. The criterion used to consider the inhibition of this response was the absence of the GMPE expression upon two consecutive stimulation periods following its repeated elicitation. At this moment, the stimulation protocol was finished.
Quantification of oscillatory variables
In order to analyse the oscillatory properties of the spinal generator for ejaculation we used electromyographic techniques to register the rhythmic activity of the bulbospongiosus muscles in the frame of the oscillatory theory proposed by Pavlidis and Pinsker [33]. The principles of this theory can be used to establish general rules concerning the working of the neuronal oscillators and their interactions with environmental stimuli [33]. Based on these principles, in the present study we defined an ejaculatory motor phase as the expression of the first motor train of a GMPE (which could be accompanied or not by an after-discharge component (Figure 1), obtained either spontaneously or in response to urethral or pharmacological stimulation. An ejaculatory period was considered as the ejaculatory capacity that permitted completing a round-trip cycle of ejaculatory motor phases previous to the inhibition of their expression.
The state variables (parameters) considered for the analysis of the oscillatory properties of the ejaculation generator were the number of discharges in the GMPE and its frequency, as well as the number of GMPE (phases) expressed prior to its inhibition. The afterdischarge component of the GMPE is only observed in urethrally evoked responses. Since this type of stimulation exerts a cumulative inhibitory effect on the expression of this after-discharge component, it was excluded from the quantitative analysis of both the ejaculatory motor phases and the periods of ejaculatory activity.
Drugs
Fluoxetine hydrochloride and urethane were purchased from Sigma Chemical Co. (St. Louis, USA) and dissolved in bidistilled water. Oxytocin was obtained from Laboratories Loffler-Mexico. Morphine sulphate was kindly donated by Laboratory Pisa-Mexico. Senecio cardiophyllus extract (SC) was prepared and administered as follows: roots of SC were collected and dried during twenty days. The dried plant material was ground into a fine powder, 100 g of which were mixed with 100 ml of distilled water. This mixture was warmed up approximately 10 min, just before boiling. The resulting extract was filtered using Whatman filter paper no. 1 and was concentrated in a rotavapor under normal pressure to obtain semisolid residues of the plant which were then lyophilised (Telstar freezer-dryer, −45 ºC). This procedure yielded 40 g of lyophilised powder. Infusions were prepared 30 min previous to p.o. administration. Doses of the drugs and plant extract were selected based on previous studies [29,32,34,35].
Experimental series
Experiment 1: Effects of sensory stimulation upon the oscillatory activity of the spinal generator for ejaculation in spinal male rats.
The objectives of these experiments were to establish the influence of sensory stimulation on the potential oscillatory activity of the spinal generator for ejaculation during an ejaculatory phase and to analyse the potential oscillations in the ejaculatory motor phases and its parameters after repeated application of the sensory stimulus. To this aim, in sexually experienced male rats (Group 1, n=5) the expression of one spontaneous ejaculatory phase was permitted after spinalisation and, after allowing a 3- min period to elapse, a GMPE was evoked by mechanical stimulation of the urethra. Thereafter, urethral stimulation was repeatedly applied, at 3- min intervals, until inhibition of the ejaculatory response.
Experiment 2: Pharmacologically-induced oscillatory behaviour of the ejaculation generator in spinal male rats.
These experimental series were aimed to analyse the potential changes in the oscillatory activity of the generator for ejaculation after pharmacologically stimulating or inhibiting its activity. In these animals, after a spontaneous GMPE was expressed, animals received an injection of oxytocin (5 I.U./rat; i.v.; Group 2, n=5) or morphine (3μg/rat; i.v.; Group 3, n=5) and the rhythmic activity and the state variables of the GMPEs, if present, were analysed. In addition, after the drug-induced response, the oscillatory ejaculatory period of these animals was evaluated by repeatedly activating the GMPE through urethral stimulation until its inhibition.
Experiment 3: Analysis of the oscillatory behaviour of the spinal generator for ejaculation in male rats of different ages.
In order to establish potential variations in the oscillatory behaviour of the ejaculation generator and their state variables in rats of different ages, the ejaculatory motor phases of neonatal rats (2-days old; Group 4, n=5) and of sexually experienced young adult (300 g; Group 5, n=5) and old (aging 2 years; Group 6, n=5) animals were repeatedly evoked by urethral stimulation and registered until its inhibition as described above.
Experiment 4: Evaluation of the oscillatory behaviour of the spinal pattern generator in male rats with distinct ejaculatory conditions.
This experiment was designed to establish putative differences in the oscillatory activity of the ejaculation generator among male rats exhibiting different ejaculatory speeds. To this purpose, young adult sexually experienced male rats with rapid (Group 7, n=5), intermediate (Group 8, n=5) or retarded ejaculation latencies (Group 9, n=5) were used to describe the oscillatory activity of the ejaculation generator by evaluating the ejaculatory motor phases expressed during repeated urethral stimulation until its inhibition.
Experiment 5: Analysis of the influence of chronic treatment with fluoxetine or Senecio cardiophyllus aqueous crude extract upon the oscillatory behaviour of the generator for ejaculation in spinal male rats.
Potential changes in the oscillatory behaviour of the spinal generator for ejaculation of male rats chronically treated with vehicle (distilled water, 1ml/kg i.p., daily for 14 days; Group 10), fluoxetine (10 mg/kg i.p., daily for 14 days; Group 11) or SC aqueous crude extract (10mg/rat p.o., daily for 8 days; Group 12) were assessed, after allowing a two-day drug holiday. Parameters of both, spontaneously expressed and urethrally-elicited ejaculatory motor phases were analysed. Finally, the oscillatory period was also evaluated in these groups.
Experiment 6: Effects of sexual training upon the oscillatory behaviour of the spinal pattern generator for ejaculation in spinal male rats.
In this experiment we provided daily sexual training to male rats to test if this manipulation induced changes in the ejaculatory motor phases, and in the ejaculatory period. In addition, we evaluated the possibility that the concomitant treatment with a pro-ejaculatory agent could positively reinforce the effects of the training procedure. To these purposes, animals were subjected daily to copulation until one ejaculation during eight consecutive days alone (intense sexual training; Group 13, n=5) or preceded by the administration of the SC crude extract (10mg/rat/day; p.o.) (Group 14, n=5). After this treatment, we permitted one day to elapse before analysing the oscillatory behaviour of the ejaculation generator during repeated activation of ejaculatory motor phases by urethral stimulation until its inhibition, as recently reported [36]. The results obtained were compared to those exhibited by sexually experienced male rats (Group 1).
Data analysis
Bulbospongiosus electromyographic activity was recorded differentially, amplified and filtered (1000x, 0.1-1 kHz band pass) (Poliview Data Acquisition System; Grass Astro-Med Inc. USA, 2003). The state variables (parameters) recorded were the number and frequency of EMG bursts. Mean values were calculated for the first GMPE from each individual manipulation (physiological-like or pharmacological). The number of ejaculatory motor phases that could be evoked prior to the inhibition of the response (ejaculatory period) was also recorded. Comparisons of the parameters obtained from spontaneous and urethrally elicited ejaculatory phases were analysed by means of the Mann-Whitney U test. A One-way ANOVA followed by the Tukey test were utilised to analyse the effects of the acute injection of oxytocin and the parameters of the ejaculatory phases of rats of different ages or sexual conditions. Data obtained from the ejaculatory phases resulting from chronic treatments were analysed by means of the Mann-Whitney U test. Finally, comparison of the effects of sexual training alone and the combined treatment with SC followed by sexual training was performed using the Twoway ANOVA followed by Holm-Sidak test. A P<0.05 was considered to be statistically significant. The Sigma Stat program (version 3.5) was used for all statistical analyses.
Results
General observations on the oscillatory behaviour of the spinal generator for ejaculation
The oscillatory properties of the spinal pattern of ejaculation were described by analysing the ejaculatory phases obtained in a spontaneous manner, as well as those elicited by urethral stimulation or induced by the systemic administration of drugs. In all studied animals, the ejaculatory motor phases elicited by stimulation to the urethra were registered as highly rhythmic motor patterns of bulbospongiosus muscle activity and included a first ejaculatory motor train and an after discharge component. These rhythmic contractions were always accompanied by the potent expulsion of seminal secretions and by penile movements including flaring, flips and cups. Consecutive repeated stimulation of the urethra induced an inhibitory effect on the ejaculatory phases, which was gradually evidenced in the parameters of the first ejaculatory motor train and in its after-discharge component. Thus, a progressive reduction in the number of motor discharges and its frequency was observed in successive ejaculatory phases. In all animals the first sensory elicited ejaculatory phase was the most potent and the last one previous to its inhibition the weakest. The oscillatory period of ejaculatory activity was completed after the expression of a mean number of six ejaculatory phases in control animals but it varied depending on the experimental manipulation the animals were subjected. Once the ejaculatory period was accomplished, no further ejaculatory phases were expressed, neither spontaneously nor in response to sensory stimulation. Similarly, no penile movements or expulsion of seminal contents occurred after the establishment of the inhibitory state. Significant differences among the ejaculatory phases elicited by urethral stimulation versus those obtained in a spontaneous manner and after the systemic administration of drugs could be noticed. Thus, the spontaneous ejaculatory phases were accompanied by penile movements including flaring, flips and cups, but the absence of the after-discharge component was always noticed. Spontaneous ejaculatory phases also lacked the presence of seminal secretions in most animals. The main feature of the drug elicited ejaculatory phases was also the absence of the after-discharge component, and when induced, these ejaculatory phases were accompanied by penile movements including flaring, flips and cups and by the potent expulsion of seminal secretions. Finally, additional differences among ejaculatory phases related to age could be noticed. Thus, in all young adult, adult and aged rats the ejaculatory phases were always accompanied by penile erections and penile movements, but in contrast, the neonatal rats did not express penile responses.
Experiment 1: The genital sensory information modulates the oscillatory activity of the spinal generator for ejaculation
In this experiment we initially compared the ejaculatory phases expressed in a spontaneous manner with those registered after the application of a first mechanical stimulation of the urethra. After the expression of the spontaneous ejaculatory phases (Figure 2A, left) sensory stimulation was applied and a facilitated ejaculatory phase was registered (Figure 2A, right). Data showed that compared to spontaneous ejaculatory phases, those sensory-evoked exhibited a statistically significant increase in the number of the ejaculatory discharges (Mann-Whitney U test, P< 0.01; Figure 3A). Urethral stimulation-induced ejaculatory phases included penile movements and expulsion of urethral contents simultaneous to the expression of individual rhythmic contractions of the muscles. The oscillatory period of ejaculatory activity was completed after the expression of a mean number of six ejaculatory motor phases. Data of this experiment were used as control data for comparison of the results obtained in experiments 2 to 6.
Experiment 2: Changes in the oscillatory activity of the generator for ejaculation induced by oxytocin and by morphine
Oxytocin (OT) injection induced the expression of ejaculatory motor phases that appeared immediately after the administration of the compound. OT-induced ejaculatory phases included individual rhythmic discharges (Figure 2B, left) concomitant to the expression of penile erections and expulsion of urethral contents. These ejaculatory phases were featured by a significant increase in the number of discharges when compared to both, control spontaneous (S) and control urethral stimulation-induced (US) ejaculatory motor phases [Tukey test, P< 0.01 and P< 0.05, respectively, Figure 3A]. A statistically significant increase in the frequency of discharge of OT-induced ejaculatory phases was also noticed when compared to spontaneous and to sensory-induced ejaculatory phases [Tukey test, P< 0.01 and P< 0.05, respectively, Figure 3B]. In addition, OT injection produced a statistically significant increase in the number of successive ejaculatory motor phases that could be evoked during an ejaculatory period in comparison with the number exhibited by control animals [Tukey test, P< 0.01, Figure 3C].
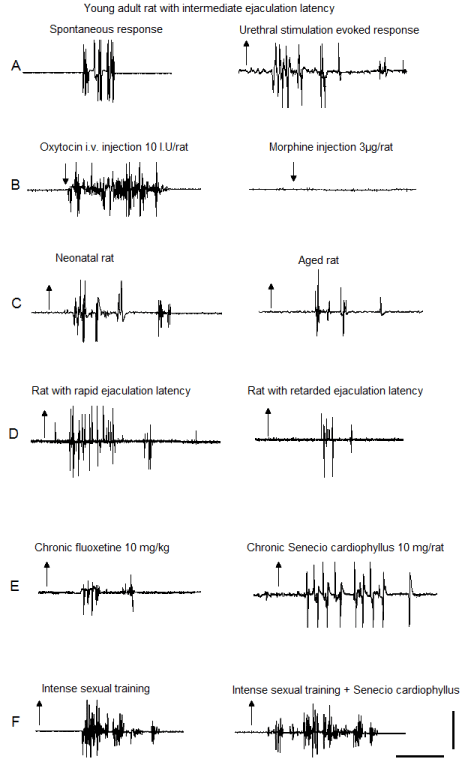
Figure 2: EMG polygraphic traces registered in the bulbospongiosus muscles
showing the oscillatory activity of the spinal generator for ejaculation in male
rats. Recordings were obtained from: (A) spontaneous (left) and sensoryinduced
(right) responses of sexually experienced young adult male rats, with
intermediate ejaculation latency; (B) after acute oxytocin (left) or morphine
(right); (C) neonatal (left) and aged (right) male rats; (D) male rats with rapid
(left) or retarded (right) ejaculatory latencies; (E) after chronic fluoxetine (left)
or SC aqueous crude extract (right); (F) rats subjected to intense sexual
training (left) and its combination with SC treatment (right). Upward arrows
indicate the mechanical stimulus “off” and downward arrows indicate drug
injection; calibration bar 50 mV, 10 s.
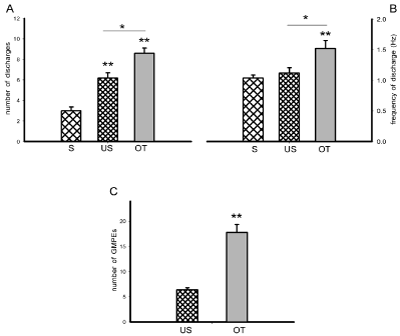
Figure 3: State variables of the ejaculatory phases (number and frequency of discharge) obtained spontaneously (S) and in response to urethral stimulation (US)
or to acute OT injection (panels A & B), and number of ejaculatory responses (GMPEs) in the oscillatory period obtained as a result of repeated urethral stimulation
in the absence or presence of OT (panel C). Values are expressed as mean ± SEM. One-way ANOVA followed by Tukey test; **P < 0.01, *P <0.05.
Morphine injection completely inhibited the expression of the ejaculatory response, i.e. motor phases and penile responses. Subsequent application of mechanical stimulation to the urethra also failed to elicit ejaculatory motor phases and penile responses (Figure 2B, right).
Experiment 3: Age-dependent changes in the oscillatory behaviour of the spinal generator for ejaculation
The ejaculatory motor phases exhibited by neonates, young adult and old male rats in response to mechanical stimulation of the urethra were all characterised by individual rhythmic contractions of the genital muscles (Figure 2A, right and Figure 2C, left & right). When comparing the ejaculatory phases and their parameters among male rats of the different ages, we observed a statistically significant reduction in the number of discharges in the ejaculatory phases expressed by aged animals as compared to both neonatal and young adult animals [Tukey test, P< 0.01, Figure 4A]. No significant differences were found in the frequency of discharge in the ejaculatory phases among neonates, young adult and aged rats (Figure 4B). A statistically significant increase in the number of ejaculatory phases in the oscillatory period was observed in aged animals when compared to the number exhibited by both neonatal and by young adult rats [Tukey test, P< 0.001, Figure 4C].
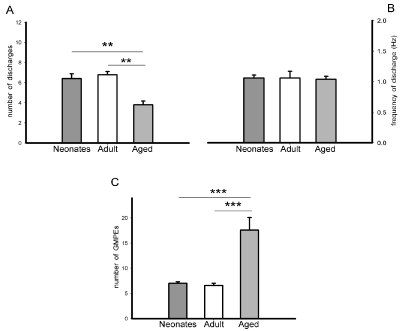
Figure 4: State variables of the ejaculatory phases (number and frequency of discharge) (panels A & B) and number of ejaculatory responses (GMPEs) in the
oscillatory period (panel C) obtained in response to urethral stimulation in neonatal, young adult and aged male rats. Values are expressed as mean ± SEM. Oneway
ANOVA followed by Tukey test; ***P <0.001, **P < 0.01.
Experiment 4: Differential oscillatory activity of the spinal generator for ejaculation of male rats with rapid, intermediate or retarded ejaculation latency
The ejaculatory motor phases in male rats with rapid (RA), intermediate (IN) and retarded (RE) ejaculation latencies could be elicited by mechanical stimulation of the urethra (Figure 2A, right and Figure 2D left & right). Comparison of the ejaculatory motor phases exhibited by the different ejaculatory subpopulations showed a diminished number of discharges in the ejaculatory motor phases of animals with retarded ejaculation latency as compared to the rats with rapid and with intermediate ejaculation latencies [Tukey test, P< 0.001, Figure 5A], while the frequency of discharge was similar in all subpopulations (Figure 5B). Differences in the ejaculatory oscillatory period were also observed in males with retarded ejaculation latency, which exhibited a statistically significantly reduced number of ejaculatory motor phases in the oscillatory period when compared to the number exhibited by both, rats with rapid and rats with intermediate ejaculation latencies [Tukey test, P< 0.001, Figure 5C].
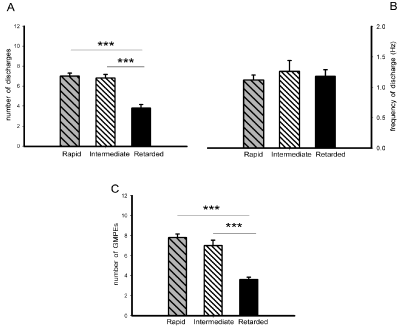
Figure 5: State variables of the ejaculatory phases (number and frequency of discharge) (panels A & B) and number of ejaculatory responses (GMPEs) in the
oscillatory period (panel C) registered in response to urethral stimulation in male rats with rapid, intermediate and retarded ejaculation latencies. Values are
expressed as mean ± SEM. One-way ANOVA followed by Tukey test; ***P < 0.001.
Experiment 5: Changes in the oscillatory activity of the spinal generator for ejaculation induced by chronic treatment (14 days) with fluoxetine or with Senecio cardiophyllus aqueous crude extract
After chronic fluoxetine (FLX) treatment, 75% of the animals exhibited spontaneous ejaculatory motor phases that were accompanied by phasic penile erections and expulsion of the urethral contents and the other 25% lacked of ejaculatory response. The spontaneous ejaculatory phases recorded were characterised by a statistically significant reduction in the number of motor discharges (Mann-Whitney U test, P< 0.05, Figure 6A left) and a similar frequency of discharge (Figure 6B left) when compared to the spontaneous responses of animals chronically injected with vehicle. Mechanical stimulation of the urethra elicited ejaculatory motor phases in all FLX- and vehicle-treated animals (Figure 2E left). The evoked ejaculatory phases of FLX-treated animals showed a statistically significant reduction in the number of motor discharges and its frequency as compared to those of vehicle-treated animals [Mann-Whitney U test, P< 0.01; Figure 6A & B, right]. Repeated urethral stimulation produced a reduced number of ejaculatory motor phases in the ejaculatory period of FLX-treated animals when compared to rats receiving vehicle [Mann-Whitney U test, P< 0.01, Figure 6C].
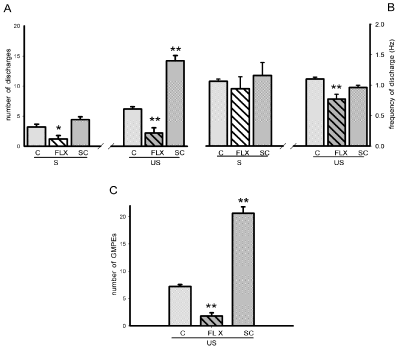
Figure 6: Effects of the chronic treatment with vehicle (C), fluoxetine (FLX) or SC upon the state variables of the ejaculatory phases (number and frequency
of discharge) registered spontaneously (S) and after urethral stimulation (US) (panels A & B), and number of ejaculatory responses (GMPEs) recorded in the
oscillatory period (panel C). Values are expressed as mean ± SEM. Mann-Whitney U test; **P <0.01, *P < 0.05 vs. vehicle-treated group.
On the other side, after chronic administration of SC aqueous crude extract all animals exhibited spontaneous ejaculatory phases that were similar in their parameters, number of discharges and frequency of discharge (Figure 6A & B left), to those exhibited by vehicle-treated animals. By contrast, the ejaculatory motor phases that were evoked by mechanical stimulation of the urethra in SC-treated rats can be seen in (Figure 2E, right) and exhibited a statistically significant increase in the number of ejaculatory discharges [Mann-Whitney U test, P< 0.01, Figure 6A, right], but not in the frequency of discharge when compared to vehicle-treated animals (Figure 6B, right). Besides, chronic SC treatment facilitated the ejaculatory oscillatory period as evidenced by the statistically significantly increased number of motor phases that could be evoked prior to the inhibition of the period [Mann-Whitney U test, P< 0.01, Figure 6C].
Experiment 6: Sexual training augments the oscillatory activity of the spinal pattern generator for ejaculation
Statistical analysis of the effects of IST alone, of SC treatment alone and of their combination on the number of discharges of spontaneous ejaculatory phases revealed significant effects of both factors, IST [F= 64.99, P< 0.001] and SC treatment [F=9.14, P< 0.01], as well as of their interaction [F=4.66, P< 0.05]. The combined treatment potentiated the facilitative effects of each individual manipulation on the state variables of the spontaneous ejaculatory phases. Thus, when comparing them with the ejaculatory phases registered in control sexually experienced animals, a statistically significant increase in the number of ejaculatory discharges was found in IST rats (Holm-Sidak test, P< 0.05). A statistically significant increase in this parameter was also found in animals receiving the combined treatment as compared both to rats subjected only to IST (Holm-Sidak test, P< 0.05) and in comparison to those receiving SC treatment alone (Holm-Sidak test, P< 0.05) (Figure 7A, left). Analysis of the effects of these experimental manipulations on the number of discharges of the ejaculatory phases evoked by urethral stimulation also showed a significant effect of IST [F= 14.39, P< 0.01], of SC treatment [F= 12.99, P< 0.01], but not of their interaction [F= ns]. A significant increase in the number of discharges of rats subjected only to IST (Figure 1F left) or treated with SC alone were found as compared to control animals (Holm- Sidak test, P< 0.05, each). The combined treatment (Figure 2F, right) significantly increased the number of discharges in the ejaculatory phases as compared to both SC (Holm-Sidak test, P< 0.05) treatment alone and IST alone (Holm-Sidak test, P< 0.05) (Figure 7A, right).
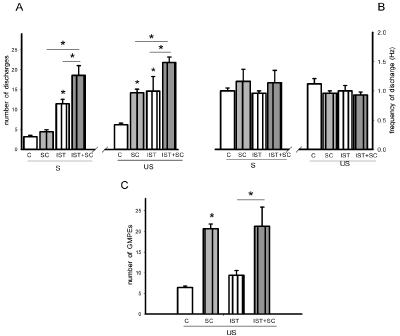
Figure 7: Effect of intense sexual training (IST), SC subchronic treatment (8 days) and of their combination (IST+SC) upon the state variables of the ejaculatory
phases (number and frequency of discharge) registered spontaneously (S) and in response to urethral stimulation (US) (panels A & B), and number of ejaculatory
responses (GMPEs) recorded in the oscillatory period (panel C). Control animals (C) are sexually experienced male rats. Values are expressed as mean ± SEM.
Two-way ANOVA followed by Holm-Sidak test; *P < 0.05.
Neither IST nor SC treatment or their combination had a significant effect on the frequency of discharge of spontaneous (Figure 7B, left) and urethral stimulation-elicited ejaculatory phases (Figure 7B, right). As to the oscillatory period of ejaculatory activity a significant effect was produced by SC treatment [F= 26.28, P< 0.001], but not by IST alone or their combination. A significant increase in the number of ejaculatory phases was observed in SC treated rats when compared to sexually experienced control rats (Holm-Sidak, P< 0.05) (Figure 7C). Similarly, males receiving the combined treatment exhibited an increased number of ejaculatory responses as compared to IST rats (Holm-Sidak, P< 0.05), but showed a similar number to that exhibited by animals treated with SC alone (Figure7C).
Discussion
The results of the present series of experiments document that the spinal generator for ejaculation in the male rat displays oscillatory behaviour that is differentially influenced by distinct stimuli and conditions. We found that:
1) genital sensory information modifies the oscillatory activity of the ejaculation circuit by activating and inhibiting its functioning; 2) the inhibitory and excitatory mechanisms mediating the oscillatory activity of the ejaculation generator can be pharmacologically turned on and shut down; 3) the oscillatory activity of the ejaculation generator varies with diverse physiological conditions like male rat’s age or ejaculatory condition (rapid, intermediate and retarded ejaculation latencies); 4) chronic treatment with specific pharmacological agents positively or negatively influences the oscillatory activity of the ejaculation generator and 5) intense sexual training exerts a notable facilitatory effect on the oscillatory activity of the ejaculation generator that can be potentiated by the concomitant treatment with SC crude extract.
As mentioned earlier, it has been shown that changes in the state variables, oscillatory periods and phases of neuronal oscillators lead to permanent changes in their oscillatory properties and motor outputs [33] and that application of sensory stimuli can induce significant changes in the oscillatory activity of a given neuronal circuit [37]. Data of the present study show that in spinal male rats, genital sensory stimulation is able to change the oscillatory activity of the generator for ejaculation by switching from single ejaculatory motor trains, seen in the spontaneous ejaculatory motor phases, to more complex ejaculatory motor responses, structured by genital motor trains with an after-discharge component, obtained in the urethrallyinduced ejaculatory responses. These data suggest that the generator for ejaculation responds to genital sensory information by adjusting its physiological properties to initiate the oscillatory activity of this circuit leading to the expression of ejaculatory trains composed by the ejaculatory motor train and its after-discharge element, similarly to the motor trains registered in normal, copulating male rats [3,23]. Thus, the genital afferent information acts at the spinal level to influence the ejaculation generator physiology switching it to a more dynamic system modulating its oscillatory capacity. Adjustments in the oscillations induced by repeated genital sensory stimulation concomitantly activate local intrinsic mechanisms, controlling the ejaculatory circuit, that initiate an intraspinal inhibitory process. This can be considered as an external, context-dependent feedback mechanism that is turned on to modulate the oscillatory behaviour of the ejaculation generator [38]. In line with this idea, it has been shown that in deafferented spinal male rats the inhibitory process does not occur and as a consequence animals are able to exhibit a high number of successive oscillatory phases for at least 2 hours without cessation [21].
Pharmacological stimuli -acutely or chronically administeredalso have complex effects on the spontaneous ejaculatory response and hence, upon the oscillatory activity of the sensory-elicited ejaculatory motor patterns. Thus, for instance, a significant increase in the activity of the spinal generator for ejaculation was induced by acute oxytocin treatment, which facilitated the oscillation of the state variables. This oscillatory activity facilitation was found to be similar to the one obtained by genital sensory stimulation, suggesting that one mechanism by which the ejaculation generator might display oscillatory behaviour is by turning on spinal oxytocinergic pathways. It has been shown that the positive influence of oxytocin on the ejaculation generator is mediated by oxytocinergic receptors located at the spinal circuit of ejaculation [38,39]. In line with this notion, receptor-mediated excitatory effects of oxytocin on the activity of spinal motor networks have also been observed [40-42]. Data of the present study show for the first time that oxytocin, a prosexual hormone commonly released during ejaculation in copulating animals [42], favours the oscillatory activity of the ejaculation generator.
Ejaculatory capacity seems to be influenced by the potential of the ejaculation generator to increase or decrease its oscillatory activity in response to excitatory and inhibitory stimuli, respectively. Thus, present data show that activation of spinal μ-opioid receptors by acute morphine, which exerts an inhibitory effect on both copulatory behaviour [43-46] and the activity of the ejaculation generator [32], also blocks the capacity of the ejaculation generator to oscillate. The fact that genital sensory stimulation, which promotes the oscillatory activity of this generator, cannot surpass the morphineinduced inhibitory state suggests that its effects are exerted at the neural elements that trigger the oscillatory activity of the ejaculation generator circuit. In addition, these data show by the first time that the opioidergic system is involved in the inhibition of oscillatory behaviour of the ejaculation generator.
Chronic fluoxetine treatment induced a significant modification of the oscillatory activity of the spinal generator for ejaculation and a significant reduction in the state variables and in the oscillatory periods. A similar result was recently reported by Hueletl-Soto et al. [35] in sexually experienced male rats chronically treated with another antidepressant, desipramine, which induced a significant modification of the oscillatory activity of the ejaculation generator, reducing one of its state variables, i.e. the number of discharges of the motor pattern and increasing the ejaculatory period, i.e. the number of successive ejaculatory motor patterns. Thus, it seems that chronic antidepressant treatment may induce sexual detrimental or facilitative effects by modifying the oscillatory activity of the ejaculation generator. This could be due to chronic drug treatment-induced neuroplastic changes that alter the functioning of the generator modifying its oscillatory behaviour. In line with this notion, chronic fluoxetine-induced neuroplastic changes include the desensitisation of 5-HT1A [47] and 5-HT1B receptors [48,49], both involved in the control of the ejaculatory threshold. More specific pharmacological studies are necessary to describe potential neuroplastic changes in the generator for ejaculation resulting from chronic drug treatments.
On the other side, chronic treatment with SC crude extract facilitated the oscillatory behaviour of the ejaculation generator augmenting the ejaculatory motor phases and facilitating its state variables by increasing its bursting activity. At present we have no explanation for the facilitative effects of this extract, but we propose that active compounds of this plant might turn on an enduring intraspinal excitatory mechanism, at the level of the ejaculation generator neurones, conditioning an increase in its oscillatory activity. Thus, it could be concluded that the components of the ejaculation generator are sensitive to plastic changes induced by the chronic presence of drugs with excitatory or inhibitory actions and therefore its functioning appears to be modifiable.
Differences in the oscillatory activity of the ejaculation generator might underlie the variations in the ejaculatory capacity of male rats of different ages. Thus, the considerable increase of the ejaculatory capacity observed in aged male rats, evidences significant changes in the oscillatory activity of the ejaculation generator as a consequence of aging. This is a striking finding since an augmented ejaculatory capacity could have been expected in young adult rats, but instead this phenomenon was observed in aged animals. Although there is no clear explanation to this finding, it might be thought that the normal inhibitory control exerted on the ejaculation generator could deteriorate as age advances, thus resulting in an indirect facilitation of the oscillatory activity of the generator in aged rats. The latter could be related to the diminution of testosterone levels, the substantial increase in androgen receptor number, and/or the concomitant increase of circulating oestrogen levels reported in aged animals [50]. In support to this notion, we have observed that the removal of sexual hormones due to castration disturbs the oscillatory capacity of the generator (unpublished results). Further experiments are necessary to analyse the possible role of hormonal variations in the changes observed in the oscillatory activity of the ejaculation generator of aged animals.
It has been proposed that endophenotypical differences underlie the variability in the ejaculation latency of both men and rats [51,52]. These differences could mainly rely on the intensity of the intraspinal inhibitory tone and on variations in the intraspinal mechanisms that control the oscillatory activity of the ejaculation generator. Thus, for instance, a more potent intraspinal inhibitory tone, acting on the oscillatory activity of the ejaculation generator, could be responsible for the delayed expression of ejaculation in retarded ejaculators. On the contrary, in rapid ejaculators, a weak inhibitory tone could allow the fast expression of ejaculation. Studies on multi unitary electrical stimulation of the spinal generator for ejaculation in rats by Borgdoff and colleagues [18] have reported differences in the threshold of GMPE expression among rats with rapid, normal and retarded ejaculation latencies, supporting a role of a differential intensity of the intraspinal inhibitory tone of the ejaculation generator neurones. Findings of the present study show additional differences of the physiological properties of the ejaculation generator of male rats with different ejaculation latencies that are centred in its oscillatory behaviour. Thus, rapid and intermediate latency ejaculators exhibited constant, stable ejaculatory phases; however rats with retarded ejaculation latency showed significantly reduced ejaculatory phases and ejaculatory capacity. These data suggest that the ejaculatory potency and capacity are significantly influenced by the oscillatory activity of the neural elements of the ejaculation generator.
The contribution of locomotor training to improve the functioning of the rhythmic motor patterns has been established. It has been demonstrated that sensory feedback mechanisms are adjustable and participate in the organisation of neural circuits in charge of movement as a result of neural training [33,53]. In addition, locomotor training studies show that the spinal plasticity, underlying the functional and structural improvement of the spinal neuronal networks responsible for inducing and maintaining rhythmicity, largely depends on the pattern of sensory afferent feedback [33,53]. In line with this notion, present findings show that intense sexual training alone or combined with a pro-ejaculatory agent treatment, favoured the ejaculatory phases, state variables and the oscillatory ejaculatory period enhancing the ejaculatory capacity. This is an important finding since it demonstrates that sexual training promotes dynamic changes in the oscillatory activity of the ejaculation generator that could result from modifications in the connectivity patterns of the neural elements of the ejaculation circuit, as a result of ejaculation itself. This finding support the idea that repeated expression of ejaculation could be considered as a physiological approach to improve the ejaculatory response in patients with poor ejaculatory fitness as reported recently [34]. In line with this notion, ejaculation has been considered as a positive reinforcer with sexual experience facilitating subsequent copulatory responses [54].
All in all, present data support the notion that the spinal generator for ejaculation acts as a neural oscillator whose activity is modified by internal and external demands. Identifying the external stimuli that challenge its activation would provide valuable information for the management of some of the ejaculatory dysfunctions.
References
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980; 210: 492–498.
- Grillner S. Neurobiological bases of the rhythmic motor acts in vertebrates. Science. 1985; 228: 143-149.
- Carro-Juárez M, Rodríguez-Manzo G. The spinal generator for ejaculation. Brain Res Rev. 2008; 58: 106-120.
- Dickinson PS. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol. 2006; 16: 604-614.
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nature Rev. 2003; 4: 573–586.
- Grillner S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron. 2006; 52: 751–766.
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006; 29: 279-306.
- Kiehn O, Quinlan KA, Restrepo CE, Lundfald Borgius L, Talpalar AE, Endo, T. Excitatory components of the mammalian locomotor CPG. Brain Res Rev. 2008; 57: 56-63.
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009; 10: 507-518.
- Grillner S. Neuronal networks in motion from ion channels to behaviour.An R Acad Nac Med (Madr). 2006; 123: 297-298.
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009; 19: 572-586.
- Brocard F, Tazerat S, Vinay L. Do pacemakers drive the central pattern generator for locomotion in mammals? Neuroscientist. 2010; 16: 139-155.
- Calabrese RL. Oscillation inn motor pattern generation networks. Curr Op Neurobiol. 1995; 5: 816-823.
- Li WC. Generation of locomotion rhythms lithout Inhibition in lertebrates: The learch for lacemaker leuros. Integr Comp Biol. 2011; 51: 879-889.
- Melamed O, Barak O, Silberberg G, Markrram H, Tsodyks M. Slow oscillations in neural networks with facilitating synapses. J Comp Neurosci. 2008; 25: 308-316.
- Grillner S, Wallén P. The ionic mechanisms underlying N-methyl-D-aspartate receptor-induced, tetrodotoxin-resistant membrane potential oscillations in lamprey neurons active during locomotion. Neurosci Lett. 1985; 60: 289-294.
- Borgdorff AJ, Bernabé J, Denys P, Alexandre L, Giuliano F. Ejaculation elicited by microstimulation of lumbar spinothalamic neurons. Eur Urol. 2008; 54: 449-456.
- Borgdorff AJ, Rössler AS, Clément P, Bernabé J, Alexandre L, Giuliano F. Differences in the spinal command of ejaculation in rapid ejaculating rats. J Sex Med. 2009; 6: 2197-2205.
- Schmidt BJ, Hochman S, MacLean JN. NMDA receptor-mediated oscillatory properties: potential role in rhythm generation in the mammalian spinal cord. Ann N Y Acad Sci. 1998; 860: 189-202.
- Yakovenko S, McCrea DA, Stecina K, Prochaska A. Control of locomotor cycle durations. J Neurophysiol. 2005; 94: 1057-1065.
- Carro-Juárez M, Rodríguez-Manzo G. Sensory and motor aspects of the coital reflex in the spinal male rat. Behav Brain Res. 2000; 108: 97-103.
- Carro-Juárez M, Rodríguez-Manzo G. Exhaustion of the coital reflex in spinal male rats is reversed by the serotonergic agonist 8-OH-DPAT. Behav Brain Res. 2001; 118: 161-168.
- Carro-Juárez M, Cruz SL, Rodríguez-Manzo, G. Evidence for the involvement of a spinal pattern generator in the control of the genital motor pattern of ejaculation. Brain Res. 2003; 975: 222-228.
- Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002; 297: 1566-1569.
- Marson L, McKenna KE. The identification of a brainstem site controlling spinal sexual reflexes in male rats. Exp Brain Res. 1990; 515: 303-308.
- Marson L., List M., McKenna K.E. Lesions of the nucleus paragigantocellularis alter excopula penile reflexes. Brain Res. 1992; 592: 187-192.
- Staudt MD, de Oliveira CV, Lehman MN, McKenna KE, Coolen LM. Activation of NMDA receptors in lumbar spinothalamic cells is required for ejaculation. J Sex Med. 2011; 8: 1015-1026.
- Durán ID, Rojas-Piloni JG, Cueva-Rolón R. Facilitation and inhibition of the urethrogenital reflex in spinal cord-transected rats. Brain Res. 1997; 775: 1-10.
- Carro-Juárez M, Rodríguez-Manzo G. Evidence for the presence and functioning of the spinal generator for ejaculation in neonatal male rats. Int J Impot Res. 2005; 17: 270-276.
- Stafford SA, Bowery NG, Tang K, Coote JH. Activation by p-chloroamphetamine of the spinal ejaculatory pattern generator in anaesthetized male rats. Neuroscience. 2006; 140: 1031-1040.
- Clement P, Peeters M, Bernabe J, Laurin M, Alexandre L, Giuliano F. Role of the neurokinin-1 receptors in ejaculation in anesthetized rats. J Sex Med. 2009; 6: 126-134.
- Carro-Juárez M, Rodríguez-Manzo G. Participation of endogenous opioids in the inhibition of the spinal generator for ejaculation in rats. J Sex Med. 2009; 6: 3045-3055.
- Pavlidis T, Pinsker HM. Oscillator theory and neurophysiology: introduction. Fed Proc. 1977; 36: 2033-2035.
- Carro-Juárez M, Alcazar C, Ballesteros-Polvo E, Villalobos-Peñalosa P. Increase of ejaculatory capacity by systemic administration of the oquichpatli (Senecio cardiophyllus) aqueous crude extract in male rats. J Ethnopharmacol. 2009; 126: 506-511.
- Hueletl-Soto ME, Carro-Juárez M, Rodríguez-Manzo G. DMI-induced sexual effects in male rats: analysis of DMI's acute and chronic actions on copulatory behavior and on the genital motor pattern of ejaculation. Pharmacol Biochem Behav. 2010; 94: 423-430.
- Rodríguez-Peña MA, Rodríguez-Manzo G, Carro-Juárez M. Ejaculatory training lengthens the ejaculation latency and facilitates the functioning of the spinal generator for ejaculation of rats with rapid ejaculation. Int J. Impot Res. 2016.
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ et al. Training locomotor networks. Brain Res Rev. 2008; 57: 241-254.
- Carro-Juárez M, Rodríguez-Manzo G. Role of the genital sensory information in the control of the functioning of the spinal generator for ejaculation. Int J Impot Res. 2005; 17: 114-120.
- Carro-Juárez M, Lobaton I, Benitez O, Espiritu, A. Pro-ejaculatory effect of the aqueous crude extract of cihuapatli (Montanoa tomentosa) in spinal male rats. J Ethnopharmacol. 2006; 106: 111-116.
- Watcho P, Carro-Juarez M. Evaluation of the ex-copula ejaculatory potentials of Bersama engleriana in spinal male rats. Asian J Androl. 2009; 11: 533-539.
- Pearson SA, Mouihate A, Pittman QJ, Whelan PJ. Peptidergic activation of locomotor pattern generators in the neonatal spinal cord. J Neurosci. 2003; 23: 10154-10163.
- de Jong TR, Veening JG, Olivier B, Waldinger MD. Oxytocin involvement in SSRI-induced delayed ejaculation: a review of animal studies. J Sex Med. 2007; 4: 14-28.
- Myers BM, Baum MJ. Facilitation by opiate antagonists of sexual performance in the male rat. Pharmacol Biochem Behav. 1979; 10: 615–618.
- Szechtman H, Hershkowitz M, Simantov R. Sexual behavior decreases pain sensitivity and stimulates endogenous opioids in male rats. Eur J Pharmacol. 1981; 70: 279–285.
- Wiesenfeld-Hallin Z, Södersten P. Spinal opiates affect sexual behavior in rats. Nature. 1984; 309: 257–258.
- Van Furth W, Wolterink-Donselaar IG, Van Ree JM. Endogenous opioids are differentially involved in appetitive and consummatory aspects of sexual behavior of male rats. Am J Physiol. 1994; 266: R606–613.
- Li Q, Muma NA, Van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther. 1996; 279: 1035-1042.
- Newman ME, Shalom G, Ran A, Gur E, Van de Kar LD. Chronic fluoxetine-induced desensitization of 5-HT1A and 5-HT1B autoreceptors: regional differences and effects of WAY-100635. Eur J Pharmacol. 2004; 486: 25-30.
- Ahlenius S, Larsson K. Evidence for an involvement of 5-HT1B receptors in the inhibition of male rat ejaculatory behavior produced by 5-HTP. Psychopharmacology (Berl). 1998; 137: 374-382.
- Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J Comp Neurol. 2009; 512: 688-701.
- Pattij T, de Jong TR, Uitterdijk A, Waldinger MD, Veening JG, Cools, AR et al., Individual differences in male rat ejaculatory behavior: searching for models to study ejaculation disorders. Eur J Neurosci. 2005; 22: 724-734.
- Olivier B, Chan JSW, Patij T, de Tong TR, Oosting RS, Veening JG et al., Psychopharmacology of male rat sexual behavior: modeling human sexual dysfunctions? Int J Impot Res. 2006; 18: S14-S23.
- Knikou M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin Neurophysiol. 2010; 121: 1655-1668.
- Powell WS, Dominguez JM, Hull EM. An NMDA antagonist impairs copulation and the experience-induced enhancement of male sexual behavior in the rat. Behav Neurosci. 2003; 117: 69-75.
