Abstract
The aim of the study was to investigate the fertility-regulating potential of the compound 2-(2″-chloroacetamidobenzyl) - 3-(3′-indolyl) quinoline or commonly called indolyl quinoline in male rats. Male rats of proven fertility were treated with the compound by oral gavages for 3, 6 and 12 weeks. Functional fertility, testicular, epididymal and seminal vesicular weight, epididymal sperm count as well as quantification of spermatogenesis process were made. Epididymis and testis samples were processed using routine techniques and prepared for light microscopy analysis in both treated and control series. Functional fertility was reduced significantly as revealed by a fall in fertility and pregnancy rate. The weight of the reproductive organs was reduced significantly. A reduction of sperm count and number of different types of testicular cells was observed. Histologically, the tested compound caused some disruption in both epididymis and testicular architecture, scanty spermatozoa and in number of differentiating spermatogenic cells. Histological alterations were assessed and the results were discussed according to the severity of the histological response made by the compound. Fertility and other effects were regained gradually after withdrawal of treatment. The results revealed from the study indicate reversible antifertility of the said chemical agent.
Keywords: Male Rattus rattus; Indolyl Quinoline; Spermatogenesis; Reproductive Organs; Anti-Spermatogenic; Reversible Antifertility
Abbreviations
GnRH: Gonadotropic-Releasing Hormone; SRL: Sisco Research Laboratory; STM: Saline-Triton-Merthiolate; ANOVA: Analysis of Variance; NS: Not Significant; GPC: glycerol Phosphorylcholine
Introduction
The population explosion is a global problem that poses significant threat to the quality of life in Third World countries. To combat this grave situation, effective family planning is required with equal participation of both males and females. An important section of reproduction is the fertility regulation by means of contraception and management of infertility [1]. Though some highly effective, acceptable and reversible contraception modalities have been developed as a contraceptive agent for the females, yet no or very little effective and reversible systemic method has been developed to date for males. Several prospective moves toward initiation of infertility using hormonal, chemical and immunological approach have been explored for a long time [2-5]. Induction of acute oligospermia or azoospermia is possible by using hormonal steroid or gonadotropic-releasing hormone (GnRH) agonist/ antagonist [6,7]. These methods are either irreversible or affect libido and secondary sex characteristics, and supplementary treatment is required appearing these methods cost-ineffective. Other way would be to find effective anti- spermatogenic agents that could rely on alternative means to the hypothalamic-pituitary gonadal axis, more particularly, compounds primarily designed for the purpose of other clinical use unrelated to contraception but unexpectedly revealed to have antifertility effect upon routine evaluation [8,9]. With the use of present-day knowledge of synthetic chemistry to synthesize different analogues and after careful pharmaco-toxicological surveillance, it is possible to achieve some useful products having no overt toxicity. Reversible antispermatogenic and antisteroidogenic properties of some traditionally used contraceptive have also been reported [10,11]. Nitrogenous heterocyclic compounds having antiflagellated or antiproliferative activity have been found to be antispermatogenic or vice versa [12]. Related compounds have been reported as antispermatogenic and have been projected as male contraceptive [2,3,13-15]. The imidazole group of compounds in clinical use against flagellated protozoa and anaerobic bacteria for genital tract infections of both men and women has been shown to have potential antifertility activity in male [16,17]. The compound 2-(2′-chloroacetamidobenzyl) - 3-(3′-indolyl) quinoline or indolyl quinoline contains indole, and quinoline moieties that match with all of the mentioned compounds [18,19]. The above information was the main impetus behind this effort of evaluating the fertility-regulating potential of the compound in male rats, including histomorphometric examination for better understanding of the spermatogenic process and testis function.
Materials and Methods
Chemicals
All chemicals and reagents were of analytical grade and were procured from Merck India Ltd. Staining reagents were from SRL India Ltd., Mumbai, India. The tested chemical compound has been synthesised through the application of Friedel-Crafts reaction [20] the purity of the compound was verified [20] and the bioactivity was determined [21].
Animals
All experiments on laboratory animals were performed as per the guidelines of the animal ethics committee of the University. Rats (Rattus rattus) from random bred colonies were housed on a fourteen hours light and ten hours dark cycle under standard husbandry conditions (temp. 220 C ±20 C, relative humidity 55 % ±5%) and were provided standard pellet food and sterile water ad libitum.
Experimental Procedure
Design of experiment, mating and fertility tests
Six different groups consisting of 5 animals in each group were allocated from a population of 60 male rats, where 50% of the total was considered as control without any treatment. The test material was administered orally by gavages to different groups of rats for 3, 6, and for 12 weeks at a dose of 250 mg/kg of body weight (5g/kg per day did not cause lethality, unpublished observation). The animals of one particular groups of a specific treatment were maintained for a period of another 3, 6 and 12 week each after discontinuation of the specific compound to find out any recovery of the effect of the test compound. Mating schedules for the experiment were planned in such a way, so that the infertility effect by the compound or the reversibility effect after discontinuation of the compound can be scrutinized accurately while monitoring their health and weight throughout the experimental period. Further the males were kept in a cage with the cyclic females (at pro estrous stage) as 1:1 ratio to observe any copulation plug or the presence of spermatozoa in the vaginal smear on the next morning and in successful mating it was considered as Day -1 of pregnancy. Accordingly, the percentage of successful mating was considered with the number of sperm positive females per number of exposed female. The so called successful mating females were separated on next morning while food and water was given ad libitum.
On day 10 of gestation, laparotomy of female rats was performed under anesthesia and their ovaries were excised, uteri were exposed including counting of corpora lutea, implantation sites and number of normal live fetuses was done.
Fertility rates were calculated by the percentage of implantation sites per number corpora lutea (representing the number of eggs ovulated) and pregnancy rate was considered to be the number of viable fetuses per female mated.
Reproductive organ weight
The males (both treated and the controls) were sacrificed after the completion of treatment schedule. The testes, epididymides and the seminal vesicles were excised, cleaned, blotted free of blood, weighed and preserved or utilized for further histological and morphological analysis.
Counting of sperm
The caudal region of epididymis was homogenized gently in a glass homogenizer in STM solution, containing 0.15 M sodium chloride, 0.05 % Triton X-100 v/v and 0.25M thimerosal. After thorough vortexing of the homogenate, one drop of the suspension was taken on the Makler counting chamber, covered with cover slip and observed under light microscope [22]. Sperms of four rows of ten squares of Makler chamber were counted. The process was repeated five times for each suspension and the average was taken and expressed per grams of epididymis.
Histological Observation
Epididymis
Epididymides of five rats per group were fixed in Bouin’s fixative, processed for histological sectioning, embedded in paraffin, and 6-μm sections were cut in a microtome and stretched on clean, grease-free glass slides. Deparaffinised sections were then processed for staining with eosin and hematoxylin and observed under microscope.
Testis
The tunica albugenia of one of the two testes was nicked at both end after immersing in Bouin’s fixative for 24 hours and continued the fixation process for another 24 hours. Tissues were dehydrated in ethanol following several changes in upgrades of alcohol, thereafter, embedded in paraffin for section cuttings and microscopic studies. The tissue slides were processed for staining through eosin - hematoxylin and finally photomicrographs were taken.
Quantification of spermatogenesis
Spermatological cells were quantified according to the method of Leblond and Clermont [23]. The relative number of each germ cell type at stage VII/VIII of the cycle of seminiferous epithelium, i.e., spermatogonia, Sertoli cells, spermatocytes and spermatid (Steps 7 and 19) was counted. The nuclear diameters of the different germ cells (Step 19 spermatids) were measured using Leitz micrometers. At least 20 round tubular cross sections were counted in each rat. All crude counts were corrected for difference in nuclear diameter by Abercrombie’s’ formula [24].
If P is the average number of nuclear points per section, A is the crude count of number of nuclei seen in the section; M is the thickness (in μ) of the section, and L the average length (in μ) of the nuclei, then
Statistical analysis
Results were expressed as mean ±standard deviation of mean of five repeated determination for 5 rats in each of the 6 groups of rats. The significance of difference in the mean data obtained were analysed and were compared using one-factor ANOVA. Values were considered significant when P<0.05 or less.
Results
Fertility status of the male rats
The fertility status of the female rats mated with a normal male rat not underwent any treatment showed a normal implantation site (Figure 1A). A significant reduction related to time of exposure in functional fertility as indicated by rate of pregnancy was noticed due to the introduction of the compound. The female rats mated with the compound treated male for 3, 6 and 12 weeks showed an average numbers (Figure 1B,1C,1D) of normal implantation sites as 4.8, 1.4 and 0.0 respectively (Table 1), whereas the females mated with control males showed number of implantation site 12.7 (Figure 1A). While the fertility rate for the control was 100% the treated group of rats showed a value ranges between 75% and 80% (Table 1). Thus, pregnancy rate of treated female animals showed decreased value compared to control group animals. Within 6 weeks of withdrawal of treatment a gradual recovery of fertility was noticed (Figure 1E,1F).
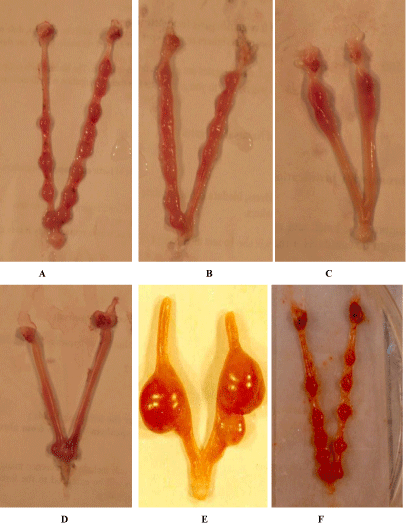
Figure 1: A. Showing normal implantation site in a normal female mated with normal male rat, B. Showing reduction of implant site in a normal female mated with
3 weeks Indolyl quinoline treated male rat, C. Showing more reduction of implant site in a normal female mated with 6 weeks Indolyl quinoline treated male rat, D.
Showing few of implant site in a normal female mated with 12 weeks indolyl quinoline treated male rat, E. Showing recovery effect of implant site in a normal female
mated with withdrawal of Indolyl quinoline after 3 weeks, F. showing recovery effect of implant site in a normal female mated with withdrawal of Indolyl quinoline
after 12 weeks.
Parameters
Experime--ntal Groups
Treatment period
Recovery period
3 weeks
6 weeks
12 weeks
3 weeks
6 weeks
12 weeks
No. of female rat mated/ No. of male rat dosed (1:1 mating)
Control
5/5
5/5
5/5
5/5
5/5
5/5
Treated
5/5
4/5
4/5
4/5
5/5
5/5
Pregnancy Percentage (%) (No. Pregnant / No. mated)
Control
100
(5/5)
100
(5/5)
100
(5/5)
100
(5/5)
100
(5/5)
100
(5/5)
Treated
80
(4/5)
75
(3/4)
80
(4/4)
75
(3/4)
80
(4/5)
100
(5/5)
No. of implantation sites
Control
13.8 1.64
12.2 0.83
14.0 1.58
12.2 1.09
11.2 1.30
12.8 ±0.83
Treated
4.800.83*
1.400.54*
0.000.00*
2.600.89#
6.401.14#
12.401.14#
Each value is expressed as the mean ±SD (n = 5 per group). Results were statistically analyzed with one-way ANOVA. *P < 0.001 compared with the control group; # P < 0.001 or P<0.01 compared with treated group.
Table 1: Percentage of pregnancy, number of implantation site of female rats mated with male treated with Indolyl quinoline at a dose of 250 mg / kg per day for 3 weeks, 6 weeks and 12 weeks along with their recovery.
Reproductive organ weight
As time went on there was a reduction in organ weight for all the three reproductive organs studied e.g. testis epididymis and the seminal vesicle compared to the weight of the reproductive organs of the control groups (Table 2). The variation in the data obtained between control and the treated groups showed significance. (Either p < 0.05 or p < 0.001). On the recovery side, the organ weight of all the organs showed a recovery in weight nearer to the normal level (Table 2) showing an intend action of withdrawal of the compound.
Parameters
Experimental
Groups
Treatment period
Recovery period
3 weeks
6 weeks
12 weeks
3 weeks
6 weeks
12 weeks
Sperm count No. X 106 / gm of Epididymis
Control
511.40 ±10.47
508 12.14
501 11.93
508.40 ±11.23
50911.66
510.611.05
Treated
439.60±7.6*
360 14.15*
1827.56*
448 14.69#
45715.59#
488.2012.33#
Organ Weight (g)
Testis
Control
3.400.02
3.500.04
3.750.18
3.410.10
3.520.34
3.690.94
Treated
2.83 0.05*
2.790.03*
3.010.15*
3.030.14#
3.080.11#
3.150.14 NS
Epididymis
Control
1.080.08
1.210.05
1.300.03
1.130.11
1.230.05
1.350.07
Treated
0.770.04*
0.770.09*
0.820.06*
0.920.08#
1.000.07#
1.120.07#
Seminal vesicle
Control
1.760.08
1.800.06
1.820.06
1.730.12
1.710.11
1.730.09
Treated
1.470.02*
1.260.07*
1.200.04*
1.320.06#
1.330.06 NS
1.360.05#
Testicular cell types (in number)
Sertoli cells
Control
5.730.07
5.690.31
6.010.91
5.730.73
5.780.24
5.920.27
Treated
4.670.12*
4.560.60*
4.810.49*
4.670.39NS
4.880.10 NS
4.950.55 NS
Pachytene spermatocytes
Control
21.661.30
21.711.84
22.082.46
22.962.07
21.751.86
22.742.49
Treated
16.930.86@
17.511.10*
18.201.27*
17.310.61#
7.911.11NS
18.121.31 NS
Round spermatids
Control
69.8310.33
70.1011.95
70.481029
69.269.59
69.238.02
71.3110.86
Treated
52.485.91@
50.104.88@
53.283.18@
59.609.87#
60.189.76#
59.679.86#
Elongated spermatids
Control
133.6010.68
134.478.54
138.057.83
135.2810.95
34.2511.22
139.1415.79
Treated
101.816.76@
80.069.39@
68.5812.44@
108.549.33#
114.65±8.41#
118.928.50#
Each value is expressed as the mean ±SD (n = 5 per group). Results were statistically analyzed with one-way ANOVA. *P < 0.01 or 0.05 compared with the control group; @ same with significant at P < 0.001.
# P < 0.01 or P<0.05 compared with treated group. NS = Not significant.
Table 2: Sperm count, organ weights, quantification of testicular cells (express/tubular section of Stage VIII/VIII after Abercrombie’s correction factor, except the elongated spermatid randomly chosen), after Indolyl quinoline treatment at different periods at a dose of 250 mg / kg per day in male rats.
Counting of sperm
The sperm count in the epididymis declined significantly (p < 0.05) within the third week of treatment by the compound and staying on towards reduction up to the twelve week (Table 2). This reduction in the number of the sperm count however gradually recovered due to the withdrawal of the treatment. At the same time no difference was observed in the morphology of the sperms under light microscope.
Epididymal histology
The epididymis in the section of the control groups revealed a lumen with normal cellular structure containing basal cells. The lumen also contains mature sperm cells (Figure 2A). The treatment with the compound after 3 weeks revealed slightly smaller tubular diameters along with thicker tubular epithelium. The number of mature sperm cells was few or scanty (Figure 2B) compared to controls. The degree of effects was very pronounced after 6 weeks (Figure 2C) and also after 12 weeks (Figure 2D) so far as the numbers of mature sperm cells are concerned. However the tubules showed more or less normal architecture of cells and comparable number of spermatozoa in the sections of epididymis of 3, 6 and 12 weeks rats after removal of the treatment. Virtually, there was no dissimilarity of the number of sperms with the control one.
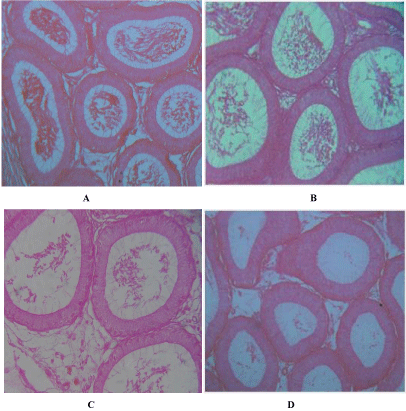
Figure 2: A. T.S. of epididymis of control rat normal architecture of tubules and spermatozoa. X 200, B. T.S. of epididymis of 3 weeks indolyl quinoline treated
male rat showing scanty spermatozoa. X 200, C. T.S of epididymis of 6 weeks indolyl quinoline treated male rat showing thicker epithelium. Note less number of
spermatozoa. X 200, D. T.S. of epididymis of 12 weeks indolyl quinoline treated male rat showing pronounced effect of the compound. Note vacuolated tubules.
X 200.
Testis histology
The section of the testis of control rats showed seminiferous tubules of variable size with spermatocytes in the lumen at different stages of maturation. (Figure 3A) The cellular interstitial connective tissue was prominent. A few blood vessels were also visible in the interstitial region. In contrast to the control the section of the testis of compound treated male rats demonstrate a structural disorganization of the seminiferous tubules along with loss in number of differentiating germinal cells either in 3 weeks or in 6 weeks of treatment (Figure 3B). In addition to, hyperpycnotic nuclei in many cells and vacuolated cytoplasm were prominent features encountered in the treated section of the testis. Exfoliated elongated spermatids were an addition to the findings. These features were very prominent in treatments after 3 weeks onwards. The tubules particularly at 12 weeks of treatment were found to be devoid of either spermatozoa or elongated spermatids (Figure 3C). The tissue sections of the testis of the treated rats showed a recovery of the germinal cells undergoing a process of differentiation after the withdrawal of the treatment for different groups of rats. However no anomalies in the morphology of sperm were noticed.
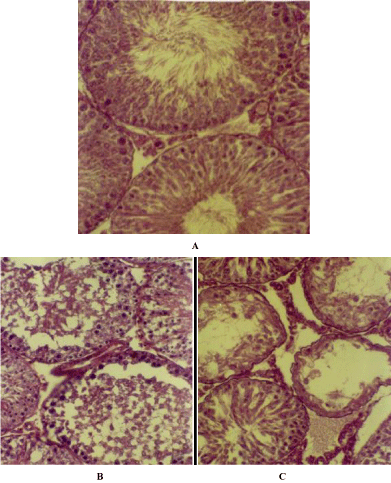
Figure 3: A. T.S. of the Testis of normal rat showing normal architecture of the seminiferous tubules with differentiating spermatogenic cells. X 200, B. T.S. of
Testis of 6 weeks indolyl quinoline treated male rat showing loss of differentiating germ cells. X 200, C. T.S. of Testis of 12 weeks indolyl quinoline treated male
rat showing vacuolated tubules. X 200.
Quantification of spermatogenesis
Though quantisation of germ cells in the testis is a contentious issue, here we followed the classical method of Leblond and Clermont [23] after adopting Abercrombie’s correction factor [24] for changes in nuclear diameter. Since all varieties of germ cells, spermatocytes of the pre-meiotic phase and the spermatids of the post meiotic phase were present the stage VII/VIII cycle of the seminiferous tubules, was chosen for quantitative study. The whole spermatogenesis process also could be judged by this study. For counting purpose, tubules having more or less cellular organization were considered. A significant (p < 0.001) decrease in number of different cell types of the treated rats, particularly affecting the pachytene spermatocytes, round and elongated spermatids (Table 2) were made known during quantification study. No distinct change was observed in Spermatogonia A, B and resting spermatocytes. Statistically significant (P < 0.05) change in the number of Sertoli cells was also observed (Table 2). The recovery experiment showed a change in the number of differentiated cell types, closer to the normal one.
Discussion
Spermatogenesis is a process of both mitotic and meiotic divisions where spermatogonia divide to produce spermatocyte and subsequently haploid spermatids. Further, the spermatids undergo a notable change during their development through a process of spermiogenesis where the round shaped structure of the spermatids turned into elongating spermatids that transform into spermatozoa through certain steps. The whole process occurs into the seminiferous tubular lumen. The compound of interest studied here is capable of inducing harm to seminiferous epithelium of rat and thus changing the process of spermatogenesis along with other reproductive parts in question causing infertility.
Functional infertility was induced in male rats by the compound through exposed time period as substantiated by reduced fertility and the rate of pregnancy. To examine the compound to have an effect on which phase of spermatogenic process, epididymal maturation and or testicular events of spermatogenesis, a periodical mating schedule followed by sacrifice of the male rats has been planned. It is known that for maturation of spermatozoa the epididymis plays a very crucial role and a cascade of reactions take place in epididymal fluid during transportation of the sperm through the convoluted ductus system of the epididymis. The fertilizing ability of the sperm [25-30] is ensured by the factors derived from the epididymal fluid that rearrange and reorganize lipids and proteins in the spermatozoa membrane [31] Decline in GPC level, an indicator of epididymal function [32], morphological change in epididymis and sperm count indicate that epididymal dysfunction could be one of the causes of infertility induced by the compound during early weeks. Moreover, no distinct morphological change in the shape and structure of the epididymal sperms as seen under light microscope indicates that the tested compound does not cause antifertility through morphological disruption of sperms.
On the other hand no significant change is observed in the mating rates between the control and treated rats, suggesting no harmful effects of the tested compound on the copulating potential of the rats. It has further been studied that the inhibition of spermatogenesis by the compound is attributed to the elevated levels of gonadotrophins (unpublished observations). The events of spermatogenesis are mainly regulated by testosterone and the role of gonadotropic also could not be ruled out [33-36]. Simultaneous testosterone administration showed prevention of degeneration of spermatogenic cells in rats at Stage VII/VIII [34,37,38].
The present morphological study shows a distinct change in number of tubular cell types with a progressive loss of differentiating cells in late stage of spermatogenesis in particular, except in spermatogonial cells. The possible cause of induction of infertility by the tested compound might be a reduction in the level of testosterone, though the other possibilities may not be ruled out (unpublished observations). The beginning and continuation of spermatogenesis in pre-pubertal and pubertal rats is LH and FSH dependant. Negative feedback regulation of FSH, LH and sex hormone to this process has also been studied extensively [39] and it has been shown that high level of FSH and LH are detrimental as sub- normal level [40,41]. Histological assessment in the present study is a useful parameter that offers a sensitive indicator of damage to the epididymis and the testis cells. The treatment made thereof through different weeks also provides information on the marked cells, degree of toxicity and also point out the probable recovery. It is known that the Sertoli cells exert a structural support in differentiation and survival of the spermatogenic germ cells within testis [42-44]. The number of Sertoli cells per testis is an indicator of sperm production and the size of the testis [45]. Similarly FSH acts as a mitotic factor [46] for Sertoli cells. Further, cooperation of testosterone and FSH brings about the binding of spermatids with the Sertoli cells. This is an indispensible step in the process spermiogenesis [47]. The present study also witness the increase in the number of Sertoli cells during recovery period which might be considered as a cause of shrinkage of tubular length, though Sertoli cells do not divide in adult under normal conditions. But it has also been shown that the terminally differentiated Sertoli cells can re-start mitotic cycle even after attaining maturity [48]. Thus it can be clearly stated that the compound imposed some change in the physiological setting of the Sertoli cells that could lead to the inhibition of spermatogenesis. Therefore, the action of the test compound on the spermatozoa pursuing epididymal maturation suggests a condition of functional infertility during the treatment period. The effects observed after withdrawal of the treatment strongly put forward the view of influential reversible antifertility activity.
References
- Allag I and Rangari K. Extra genomic action of steroids on spermatozoa Prospects for regulation of fertility. Health Popul. 2002; 25: 38-44.
- Gottwald U, Davies B, Fritsch M, Habenicht U. New approaches for male fertility control: HE6 as an example of a putative target. Mol Cell Endocrinol. 2006; 250: 49-57.
- Kopf GS. Approaches to the identification of new nonhormonal targets for male contraception. Contraception. 2008; 78: S18-22.
- Etribi A, Ibrahim A, Mahmoud K, El-Haggar S, Hamada T, El-Ahmadi I. Antisperm antibodies and human infertility. Fertil Steril. 1982; 37: 236-239.
- Vannucchi C, Angrimani D, Eyherabide A, Mazzei C, Lucio C, Maiorka P, et.al . Effects of intratesticular administration of zinc gluconate and dimethyl sulfoxide on clinical, endocrinological, and reproductive parameters in dogs. Theriogenology. 2015; 84: 1103-1110.
- Paulsen C, Christensen R, Bagatell C. Status of male contraception: hormonal approach. In: Corson SL, Derman RJ, Tyner LB, editors. Fertility control. 2nd ed. London: Golden Publishers; 1994: 281–292.
- Edwards B, Smith A, Skinner DC. Dose and durational effects of the gonadotrophic-releasing hormone agonist, deslorelin: the male rat (Rattus norvegicus) as a model. J Zoo Wildl Med. 2013; 44: S97-101.
- Corsi G, Palazzo G, Germani C, Barcellona S, Silvestrini B. 1-Halobenzyl-1H-indazole –3-carboxylic acids. A new class of antispermatogenic agents. J Med Chem. 1976; 19: 778–783.
- Silvestrini B, Palazzo G, De Gregario M. Lonidamine and related compounds. Prog Med Chem. 1984; 21: 110–135.
- Dhanapal R, Ratna, JV, Saratchandran I, Gupta M. Reversible antispermatogenic and antisteroidogenic activities of Feronia limonia fruit pulp in adult male rats. Asian Pac J Trop Biomed. 2012; 2: 684-690.
- Mishra RK, Singh SK. Reversible antifertility effect of aqueous rhizome extract of Curcuma longa L. in male laboratory mice. Contraception. 2009; 79: 479-487.
- 12. Grima J, Silvestrini B, Cheng Y. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2, 4′-dichlorobenzyl) - indazole-3-carbohydrazide. Biol Reprod. 2001; 64: 1500–1508.
- Singla N, Kaur G, Babbar BK, Sandhu BS. Potential of triptolide in reproductive management of the house rat, Rattus rattus. Integr Zool. 2013; 8: 260-276.
- Gupta RS, Saxena P, Gupta R, Kachhawa JB. Evaluation of reversible contraceptive activities of Cuminum cyminum in male albino rats. Contraception. 2011; 84: 98-107.
- Lohiya NK, Mishra PK, Pathak N, Manivannan B, Bhande SS, Panneerdoss S, Sriram S. Efficacy trial on the purified compounds of the seeds of Carica papaya for male contraception in albino rat. Reprod Toxicol. 2005; 20: 135-148.
- Cooper TG, Yeung CH, Skupin R, Haufe G. Antifertility potential of ornidazole analogues in rats. J Androl. 1997; 18: 431–438.
- Chakrabarty G, Basu A, Manna PP, Mahato SB, Mandal NB, Bandyopadhyay S. Indolylquinoline derivatives are cytotoxicity to Leishmania donovani promastigotes and amastigotes in vitro and are effective in treating murine visceral leishmaniasis. J Antimicrob Chemother. 1999; 43: 359–366.
- Grima J, Silvestrini B and Cheng Y. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2, 4¢-dichlorobenzyl)- indazole-3-carbohydrazide. Biol Reprod. 2001; 64: 1500-1508.
- Bone W, Jones NG, Kamp G, Yeung CH, Cooper TG. Effect of ornidazole on fertility of male rats: inhibition of a glycolysis-related motility pattern and zona binding required for fertilization in vitro. J Reprod Fertil. 2000; 118: 127–135.
- Mahato SB, Mandal NB, Chattopadhyay S, Nandi G, Luger P, Weber M. Synthesis of indolylquinolines under Friedel–Crafts reaction conditions. Tetrahedron. 1994; 50: 10803–10812.
- Bhowal SK, Lala S, Hazra A, Paira P, Banerjee, Mondal NB, Chakraborty S. Synthesis and assessment of fertility-regulating potential of 2-(2″-chloroacetamidobenzyl)-3-(3′-indolyl) quinoline in adult rats as a male contraceptive agent. Contraception. 2008; 77: 214–222.
- Makler A. The improved ten micrometer chamber for rapid sperm count and motility evaluation. Fertil Steril. 1980; 33: 337–338.
- Leblond CP, Clermont Y. Definition of the stages of cycle of the seminiferous epithelium in the rat. Ann N YAcad Sci. 1952; 55: 548–573.
- Abercombie M. Estimation of nuclear population from microtome sections. Anatom Rec. 1946; 94: 239–247.
- Robaire B, Viger RS. Regulation of epididymal epithelial cell function. Biol Reprod. 1995; 52: 226–236.
- Dacheux JL, Gatti JL, Dacheux F. Contribution of epididymal secretary proteins for spermatozoa maturation. Microsc Res Tech. 2003; 61: 7–17.
- Gatti JL, Castella S, Dacheux F, et al. Post-testicular sperm environment and fertility. Anim Reprod Sci. 2004; 82-83: 321–339.
- Turner TT, Miller DW, Avery EA. Protein synthesis and secretion by the rat caput epididymis in vivo: influence of the luminal microenvironment. Biol Reprod. 1995; 52: 1012–1019.
- Metayer S, Dacheux F, Dacheux JL, Gatti JL. Comparison, characterization, and identification of protease and protease inhibitors in epididymal fluids of domestic animals. Matrix metalloproteinases are major fluid gelatinase. Biol Reprod. 2002; 66: 1219–1229.
- Blobel CP. Functional processing of fertilin; evidence for a critical role proteolysis in sperm maturation and activation. Rev Reprod. 2000; 5: 75–83.
- Yanagimachi R. Fertility of mammalian spermatozoa: its development and relativity. Zygote. 1994; 2: 371–372.
- Setchel BP, Maddocks S, Brooks DE. Anatomy, vasculature, enervation and fluids of the male reproductive tract. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York Raven Press. 1994; p: 1063–1175.
- Clearmont Y, Hervay SG. Duration of the cycles of the epithelium of normal, hypophysectomized and hypophysectomized hormone treated albino rats. Endocrinology. 1965, 76: 80–89.
- Meitrich ML, Wilson G, Ye WS, Kurdglu B, Parchuri N, Terry NHA. Hormonal protection from procarbazine-induced testicular damage is selective for survival and recovery of stem spermatogonia. Cancer Res. 1994; 54: 1027–1034.
- McLachlan RI, Wereford NG, deKrester M, Robertson DM. Testosterone effect on spermatogenesis in the gonadotropin-releasing hormone immunized rat. Biol Reprod. 1994; 50: 271–280.
- O’Donnell L, McLachlan RJ, Wereford NG. Testerone withdrawal promotes stage specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996; 55: 895–901.
- McLachlan RI, Wereford NG, Tgouts C, de Krester M, Robertson DM. Effects of testosterone on spermatogenic cell populations in the adult rat. Biol Reprod. 1994, 51: 945–955.
- McLachlan RI, Wereford NG, Donnel L, de Krester M, Robertson D. The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J Endocrinol. 1996, 148: 1–9.
- Everett JW. Pituitary and hypothalamus: perspective and overview. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York Raven Press. 1994; p: 1509–1526.
- Kerr JB, Sharpe RM. Focal disruption of spermatogenesis in testis of adult rats after a single administration of human chorionic gonadotrophin. Cell Tissue Res. 1989; 257: 163–169.
- Sharpe RM. Toxicity of spermatogenesis and its detection. In: Kenneth SK, editor. Reproduction and development. New York Marcel Dekker Inc. 1994; p: 625–635.
- De Krester DM, Kerr JB. The cytology of testis. In: Knobil E, Neill JD, editors. The physiology of reproduction, vol. 1. New York Raven Pres. 1988; p: 887–932.
- Bardin CW, Cheng CY, Musto NA, Gunsalus GL. In: Knoboil E, Neill JD, editors. The Sertoli cells. New York Raven Press; 1988; P: 933–74.
- Griswold MD. Protein secretion by Sertoli cells. In: Russell LD, Griswold MD, editors. The Sertoli cells. Clearwater (FL): Cache River Press. 1993; p: 195–200.
- Orth JM, Gunsalus GL, Laperti AA. Evidence from Sertoli cell depleted rats indicates that spermatids in adults depend on the number of Sertoli cells produced during perinatal development. Endocrinology. 1998; 122: 787–794.
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill EK, editors. The Physiology of Reproduction. New York Raven Press. 1994; P: 1363–1434.
- Muffy KE, Nazian S, Cameron DF. Junction-related Sertoli cell cytoskeleton in testosterone-treated hypophysectomized rats. Biol Reprod. 1993; 49: 1122–1132.
- Chaudhury I, Sadler-Rggleman I, Ague JM, Skinner MK. The helixloop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated Sertoli cell to re-enter the cell cycle and Proliferate. Biol Reprod. 2005; 72: 1205–1217.
