Abstract
Erectile dysfunction (ED), a very frequent finding among type 2 diabetes patients (T2DM), is associated with cardiovascular disease. To investigate the prevalence of ED among our T2DM population and its association with microangiopathic complications (diabetic retinopathy (DR) and microalbuminuria [mAlb]), we performed a retrospective cross-sectional study involving 121 patients attending the Diabetology Unit of Padua Hospital. All subjects were studied with accurate anamnesis, IIEF-5 questionnaire, microalbuminuria determined in spot urine sample, fundus examination and carotid artery echo-color-doppler. ED prevalence was 64.8% while DR and mAlb prevalence was 25.6% and 23.1% respectively. In ED group vs. non-ED, DR prevalence was 32.9% vs. 11.9% (p=0.012) and mAlb prevalence was 26.6% vs. 16.7% (p=0.218). ED group had a worse glycemic control (HbA1c 7.6 ± 1.6 vs. 7.0 ± 1.0 %, p=0.010) and a longer T2DM duration (10.3 ± 9.2 vs. 6.0 ± 5.7 years, p=0.002). Furthermore, ED was associated with a higher carotid intima-media thickness (IMT 0.9 ± 0.2 vs. 0.8 ± 0.2 mm, p=0.049). ED was the first vascular complication in 57% of patients, occurring some years before DR and mAlb. Association with DR and mAlb is independent of common cardiovascular risk factors. In conclusion, ED onset in diabetic subjects is a very important finding that can be considered an early microangiopathic marker in T2DM subjects, suggesting the evaluation for the presence of other microangiopathic complications and a more intense control of cardiovascular risk factors.
Keywords: Erectile Dysfunction; Diabetes; Diabetic Retinopathy; Microalbuminuria; Cardiovascular Disease; Nitric Oxide
Abbreviations
ED: Erectile Dysfunction; T2DM: Type 2 Diabetes Mellitus; DR: Diabetic Retinopathy; Malb: Microalbuminuria; DN: Diabetic Neuropathy; IIEF-5: International Index Of Erectile Function – 5; MI: Myocardial Infarction; CVD: Cardiovascular Disease; CHD: Coronary Heart Disease; Hba1c: Glycated Hemoglobin; LH: Luteinizing Hormone; FSH: Follicle-Stimulating Hormone; E2: Estradiol; PSA: Prostatic-Specific Antigen; Egfr: Estimated Glomerular Filtration Rate; LDL: Low-Density Lipoprotein; HDL: High-Density Lipoprotein; IMT: Intima-Media Thickness; BMI: Body Mass Index; NO: Nitric Oxide
Introduction
Type 2 diabetes mellitus (T2DM) is not merely a disorder of carbohydrate metabolism, but a cause of vascular diseases affecting nearly all arterial vessels which are classically divided in microangiopathic and microangiopathic. The link between diabetes and macroangiopathic disease was suggested many years ago, observing a higher risk of myocardial infarction (MI) and cardiovascular death in several diabetic populations. In Italy, diabetic patients have a cardiovascular mortality excess of about 30-40% vs. non diabetic individuals [1-3]. Microangiopathic disease is characterized by three major manifestations: diabetic retinopathy (DR), diabetic neuropathy (DN) and diabetic nephropathy. Around 30% of diabetic patients suffer from DR, ranging from mild to severe. Male sex, higher glycated haemoglobin levels, longer duration of diabetes mellitus, higher blood pressure values and use of insulin are all associated with the development of retinopathy [4,5]. Diabetic nephropathy in T2DM occurs in 20-40% of patients and microalbuminuria (mAlb) is a marker of early nephropathy [6,7]. DR and mAlb are both associated with an increased incidence of cardiovascular disease (CVD) and mortality [4,7]. Erectile dysfunction (ED), defined as the persistent inability to achieve or maintain penile erection sufficient for satisfactory sexual performance [8], may be the first sign of CVD. Montorsi et al. have shown that ED can precede coronary and peripheral artery disease of some years [9] and a relationship between ED and silent myocardial ischemia has been demonstrated in apparently uncomplicated T2DM patients [10]. ED has a much higher prevalence among diabetic vs. non-diabetic subjects [11]. A large Italian case study observed a prevalence of 35.8% [12], while others between 35 and 90% [13]. The ADVANCE study (Action in diabetes and Vascular Disease: Preterax and Diamicron Modified- Release Controlled Evaluation) showed that in diabetic patients the presence of ED at the enrolment was associated with a high risk for all cardiovascular events, coronary heart disease (CHD) and cerebralvascular disease [14]. To our knowledge, no data is still available about onset precocity of ED among microangiopathic complications, therefore we investigated the prevalence of ED among our T2DM population, its association with micro-vascular complications (DR and mAlb) and their occurrence timing.
Materials and Methods
This is a retrospective cross-sectional study, involving 121 patients attended at the Diabetology Unit of Padua Hospital, who followed a normal screening schedule for the complications of T2DM. All patients were T2DM patients and had an age between 40 and 78 years with a mean age of 58.2 ± 8.5 years, mean T2DM duration of 8.8 years, BMI 29.0 ± 4.7 Kg/m2 and glycated haemoglobin (HbA1c) 7.5 ± 1.4% (Table 1). All the subjects underwent an accurate medical history collection including ongoing therapy, International Index of Erectile Function (IIEF-5) questionnaire, physical examination (weight, height, BMI, waist circumference and blood pressure), biochemical blood tests (fasting plasma glucose, glycated haemoglobin, total cholesterol, high-density lipoprotein cholesterol, triglycerides, and creatininemia), hormone levels (LH, total testosterone, estradiol (E2) and PSA). Blood collection and pressure measurements were performed in fasting condition, between 08.00 and 10.00 a.m., avoiding cigarette smoking for a minimum of 12h. Blood samples were collected in SST II, LHPST II, and EDTA tubes and analysed concurrent to blood draw. We excluded patients with post-surgical ED, neoplastic patients, with end-stage renal or liver insufficiency and transplanted patients.
All subjects (n 121)
Non-DE (n)
DE (n)
P
Age (years)
58.2 ± 8.5
56.5 ± 6.7
59.2 ± 9.2
0.072
T2DM duration (years)
8.8 ± 8.6
6.0 ± 5.7
10.3 ± 9.5
0.002
Fasting plasma glucose (mg/dl)
157 ± 52
142.4 ± 33.7
165.2 ± 58.1
0.007
HbA1c (%)
7.5 ± 1.4
7.0 ± 1.0
7.6 ± 1.6
0.010
BMI (Kg/m2)
29.0 ± 4.7
28.0 ± 3.4
29.5 ± 5.3
0.091
Overweight/obesity (%)
81
78.5
83.6
0.765
Waist circumference (cm)
101.8 ± 10.7
99.4 ± 8.5
103.1 ± 11.6
0.052
Systolic blood pressure (mmHg)
134.7 ± 18.9
132.0 ± 18.1
136.1 ± 19.4
0.258
Diastolic blood pressure (mmHg)
80.1 ± 10.4
79.6 ± 11.3
80.3 ± 9.9
0.735
Hypertension (%)
51.2
45.2
54.4
0.348
Smokers (%)
62.6
59.4
64.8
0.648
Total cholesterol (mg/dl)
178.7 ± 38.1
172.1 ± 31.5
182.3 ± 41.0
0.162
HDL-cholesterol (mg/dl)
45.7 ± 12.1
46.4 ± 10.2
45.4 ± 13.1
0.687
non-HDL cholesterol (mg/dl)
133.0 ± 37.5
125.7 ± 30.6
136.9 ± 40.3
0.120
Triglycerides (mg/dl)
149.4 ± 108.9
130.8 ± 68.6
159.3 ± 124.5
0.172
LDL-cholesterol (mg/dl)
103.1 ± 31.9
99.6 ± 26.2
105.0 ± 33.2
0.360
Creatininemia (mmol/l)
80.8 ± 20.1
78.3 ± 15.3
82.2 ± 22.2
0.311
eGFR (CKD, ml/min)
120.3 ± 23.5
93.2 ± 13.9
88.4 ± 17.7
0.129
LH (UI/l)
6.2 ± 3.6
5.9 ± 4.0
6.3 ± 3.4
0.582
Testosterone (nmol/l)
13.3 ± 6.5
12.0 ± 6.4
13.9 ± 6.5
0.220
Estradiol (pmol/l)
86.1 ± 40.3
67.0 ± 29.9
92.0 ± 42.3
0.102
PSA (ng/ml)
1.33 ± 1.43
1.4 ± 1.3
1.3 ± 1.6
0.780
Carotid IMT (mm)
0.87 ± 0.34
0.8 ± 0.2
0.9 ± 0.2
0.049
Table 1:
The presence of ED and age of its onset was assessed with anamnesis together with IIEF-5 questionnaire for ED. Urinary albumin excretion rate was determined in three or more samples of spot urine within six months, and considered pathological when >30mg/gcreatininuria (mAlb), DR was screened and staged through fundus examination. Furthermore, in the context of the general T2DM complication screening, patients underwent a carotid US color-doppler. Patients have been considered “smokers”, if active smokers or former smokers, we assumed a cut-off for hypertension of a systolic blood pressure >140mmHg and/or a diastolic blood pressure >90mmHg, hypercholesterolemia was defined as LDLcholesterol >100mg/dl or non-HDL cholesterol >130mg/dl, the estimated glomerular filtration rate was calculated using the CKDEPI equation.
Data are expressed as mean ± standard deviation for continuous variables or as N with percentage for categorical variables. For comparison, a two-sample t-test was performed to identify the differences of continuous variables between the ED and non-ED groups, whereas the Pearson X² test was performed for categorical variables between the ED and non-ED groups. Multiple analysis of covariance (MANCOVA) was applied to correct for DM duration and HbA1c the differences found between the ED and non-ED groups. The level of significance was considered as P <0.05. Statistical analysis was performed using SPSS statistics software for Windows version 23.
Results and Discussion
In our T2DM population we found a high prevalence of ED that reached 64.8% (79 patients). DR prevalence was 25.6% in the whole cohort, being significantly higher in the ED group than in non-ED group (32.9 vs. 11.9%, p=0.012) (Figure 1A). mAlb prevalence was 23.1%, and similarly to RD, higher in patients with ED than in non- ED subjects (26.6 vs. 16.7%, p=0.218) (Figure 1B). The ED group had a worse glycemic control, as demonstrated by higher fasting plasma glucose, and HbA1c levels, and presented a longer T2DM duration (Table 1). On the other hand, other studied parameters, as age, smoke, overweight/obesity (i.e. BMI >25Kg/m2), waist circumference, arterial hypertension, serum lipid profile, eGFR, and hormones did not differ between ED and non-ED group (Table 1). As regards ongoing therapy, data did not show any difference between ED and non-ED group.
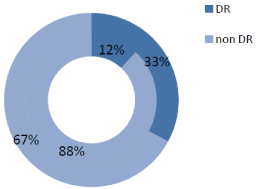
Figure 1A: DR Prevalence in DE group vs. Non DE group.
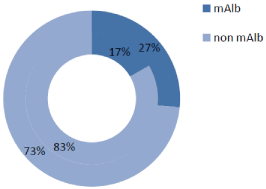
Figure 1B: mAIb prevalence in DE group vs. Non DE group.
Moreover ED was associated with morphological alterations in the carotid artery and specifically with a higher intima-media thickness (IMT 0.9 ± 0.2 vs. 0.8 ± 0.2 mm), this finding remained statistically significant after adjustment for T2DM duration and metabolic control (p=0.049).
Finally, we were able to establish the onset timing of each microangiopathic complication. ED occurred first in a large majority of patients (57% of the patients), DR was the first microangiopathic complication in 16%, and mAlb in 15% of the subjects. When ED occurs first, it anticipates on average the onset of mAlb and the diagnosis of DR by 30 months. Statistical analysis showed that, when ED occurs first, this is independent of risk factors such as hypertension (p=0.457), hypercholesterolemia (p=0.572), smoke (p=0.403), overweight/obesity (i.e. BMI >25Kg/m2, p=0.374) or poor glycemic control (i.e. HbA1c >7.5%, p=0.930).
In our diabetic cohort we found a statistically significant association between ED and DR, we also observed a higher prevalence of pathological albuminuria in the ED group vs. non-DE group, although this trend did not reach the statistical significance, probably as a consequence of the physiological wide variability of urinary albumin excretion [15] or because of the numerosity cohort considered. These findings are consistent with previous data that showed an association between diabetic retinopathy and erectile dysfunction in type 2 diabetics [16,17] and a relationship between ED end albuminuria in T2DM men [18] and recognizing albuminuria as an independent risk factor of erectile dysfunction in T2DM patients [19]. Furthermore, ED is usually the first chronic complications of DM to appear, and it may anticipate by years the diagnosis of the other microangiopathic complications.
Erectile dysfunction is defined as the persistent inability to achieve or maintain penile erection sufficient for satisfactory sexual performance [8]. Erection is a complex mechanism in which there is an increase in blood flow to the corpora cavernosa, which occludes venous blood efflux and thereby raises intracavernous pressure to ensure a firm erection. This hemodynamic phenomenon results from the interplay of neurological, endocrinological, tissutal, psychological, relational and vascular factors. In particular, endothelial-derived nitric oxide (NO) is probably the most important mediator of vasodilatation, and endothelial dysfunction, affecting NO release, is crucial to vasodilatation impairment in ED [20]. Actually, ED is in 70% of cases vasculogenic [21] in part as a consequence of endothelial dysfunction.
Among diabetic patients there is a high prevalence of ED [12]. Diabetes can affect erection at different levels, essentially through hormones derangement, neuronal impairment, local factors and, above all, vasculopathy [13]. As regards vasculopathy, we already know that diabetes mellitus associates with vascular diseases classically divided in macroangiopathic and microangiopathic. Among microangiopathic complications we recognize diabetic nephropathy, revealed by its early marker microalbuminuria, and diabetic retinopathy. In our population we found an association between ED and DR and also a higher prevalence of mAlb among ED patients. A possible explanation for this clustering of microangiopathic complications could be a generalized endothelial dysfunction which is a hallmark of both mAlb [22] and DR [16]. Endothelial dysfunction is characterized by a reduced vasodilatation in response to stimuli, procoagulation, inflammation and arterial stiffness [23]. Many factors in diabetes promote endothelial dysfunction, which has been already well documented both in diabetic [24] and in non-diabetic obese subjects [25]. ED is a well established marker of diffuse endotheliopathy, a condition common to diabetes, and the physiopathological pathway for the development of its complications, microalbuminuria [26] and diabetic retinopathy [27].
Conversely, the higher prevalence of DR and mAlb in the DE group we observed in our population is not attributable to differences in common cardiovascular risk factors like smoke, higher BMI, waist circumference, hypertension, dislipidemia, renal function, and hormones. On the other hand, we find a correlation between ED and a longer DM duration and a worse metabolic control confirming the importance of hyperglycaemia in the pathogenesis of DE as already demonstrated in other studies [28].
Given this association between microangiopathic complications and ED, we also investigated which one occurred first in our cohort, in order to identify the more precocious microangiopathic manifestation. Our results show that ED occurs some years before the other microangiopathic complications in most cases. ED may thus be considered a very early marker of microangiopathic disease suggesting a more intensive control of cardiovascular risk factors and further evaluation for the presence of other microangiopathic complications.
The validity of ED as an early marker of initial vascular disease is confirmed by its significant association with a higher IMT value in the carotid arteries, as already demonstrated in previous studies in non-diabetic subjects [29].
Conclusion
ED is a very frequent finding among T2DM patients, and associates with other microangiopathic complications, such as DR and early diabetic nephropathy (i.e. microalbuminuria), this association is independent of other classical risk factors, like hypertension, hypercholesterolemia or overweight/obesity. Among microangiopathic complication, in more than one half of our patients, ED occurred some years before the others. ED onset in diabetic patients is hence a very important finding concerning cardiovascular risk because it can anticipate DR and mAlb. ED can therefore be considered an early microangiopathic marker in T2DM subjects, suggesting the evaluation for the presence of other microangiopathic complications and a more intense control of cardiovascular risk factors.
References
- Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998; 339: 229-234.
- Bruno G, Merletti F, Boffetta P, et al. Impact of glycaemic control, hypertension and insulin treatment on general and cause-specific mortality: an Italian population-based cohort of type II (non-insulin-dependent) diabetes mellitus. Diabetologia. 1999; 42: 297-301.
- Brun E, Nelson RG, Bennett PH, et al. Verona Diabetes Study. Diabetes duration and cause-specific mortality in the Verona Diabetes Study. Diabetes Care. 2000; 23: 1119-1123.
- Yau JW, Rogers SL, Kawasaki R, et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care. 2012: 35: 556-564.
- Guo VY, Cao B, Wu X, Lee JJ, Zee BC. Prospective Association between Diabetic Retinopathy and Cardiovascular Disease-A Systematic Review and Meta-analysis of Cohort Studies. J Stroke Cerebrovasc Dis. 2016; 25: 1688- 1695.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of micro albuminuria is associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004; 110: 32-35.
- Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010; 375: 2073-2081.
- NIH Consensus Conference Impotence NIH Consensus Development Panel on Impotence. JAMA. 1993; 270: 83-90.
- Montorsi P, Ravagnani PM, Galli S, Salonia A, Briganti A, Werba JP, et al. Association between erectile dysfunction and coronary artery disease. Matching the right target with the right test in the right patient. Eur Urol. 2006; 50: 721–731.
- Gazzaruso C, Giordanetti S, De Amici E, Bertone G, Falcone C, Geroldi D, et al. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. Circulation. 2004; 110: 22-26.
- Hatzimouratidis K, Amar E, Eardley I, et al. European Association of Urology. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010; 57: 804-814.
- Fedele D, Coscelli C, Santeusanio F, et al. Erectile dysfunction in diabetic subjects in Italy. Gruppo italiano studio deficit erettile nei diabetici. Diabetes Care. 1998; 21: 1973-1977.
- Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009; 6: 1232–1247.
- Batty GD, Li Q, Czernichow S, Neal B, et al. ADVANCE Collaborative Group. Erectile dysfunction and later cardiovascular disease in men with type 2 diabetes: prospective cohort study based on the ADVANCE (Action in diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation) trial. J Am Coll Cardiol. 2010; 56: 1908-1913.
- McFarlane PA. Testing for albuminuria in 2014. Can J Diabetes. 2014; 38: 372-375.
- Chew SKH, Taouk Y, Xie J, Nicolaou TE, Wang JJ, Wong TY, et al. Relationship between diabetic retinopathy, diabetic macular oedema and erectile dysfunction in type 2 diabetics. Clinical and Experimental Ophthalmology. 2013; 41: 683-689.
- Henis O, Shahar Y, Steinvil A, Finn T, Heruti R, Loewenstein A, et al. Erectile dysfunction is associated with severe retinopathy in diabetic men. Urology. 2011; 77: 1133-1136.
- Yu LW, Kong AP, Tong PC, Tam C, Ko GT, Ho CS, et al. Evaluation of erectile dysfunction and associated cardiovascular risk using structured questionnaires in Chinese type 2 diabetic men. Int J Androl. 2010; 33: 853- 860.
- Chuang Y, Chung M, Wang P, Lee W, Chen C. Albuminuria is an independent risk factor of erectile dysfunction in men with type 2 diabetes. J Sex Med. 2012; 9: 1055-1064.
- Maas R, Schwedhelm E, Albsmeier J, Boger RH. The pathophysiology of erectile dysfunction erlated to endothelial dysfunction and mediators of vascular function. Vasc Med. 2002; 7: 213-215.
- Lue TF, ed impotence and infertility. In: Vaughan ED Jr. Perimutter AP, eds. Atlas of Clinical Urology. Vol 1. Philadelphia, Pa: Current Medicine. Inc 1999.
- Deckert T, Feldt-Rasmussen B, Bprch-Johnsen K, Jensen T, Kofoed- Enecoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989; 32: 219-226.
- Hamilton SJ, Watts GF. Endothelial dysfunction in diabetes: pathogenesis, significance, and treatment. Rev Diabet Stud. 2013; 10: 133-156.
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, et al. Impaired endothelium dependent and independent vasodilatation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992; 35: 771–776.
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996; 97: 2601–2610.
- Baron AD, Steinberg HO. Endothelial function, insulin sensitivity, and hypertension. Circulation. 1997; 96: 725–726.
- Parving HH, Gall MA, Skøtt P, Jørgensen HE, Løkkegaard H, Jørgensen F, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992; 41: 758-762.
- Ryu JK, Jin HR, Yin GN, Kwon MH, Song KM, et al. Erectile dysfunction precedes other systemic vascular diseases due to incompetent cavernous endothelial cell-cell junctions. J Urol. 2013; 190: 779-789.
- Foresta C, Palego P, Schipilliti M, Selice R, Ferlin A, et al. Asymmetric development of peripheral atherosclerosis in patients with erectile dysfunction: an ultrasonographic study. Atherosclerosis. 2008; 197: 889-895.
