Abstract
The prevalence of skin diseases in men with Klinefelter syndrome in general is identical to that in men with normal genotype. However, there are some typical diseases which occur with enhanced frequency in Klinefelter syndrome. These include X-linked genodermatoses, which are normally lethal in male offspring, leg ulcer in younger men, male breast cancer and gynecomastia. The clinical features of these diseases are described in the paper.
Keywords: X-linked genodermatoses; Leg ulcer; Morbus Paget; Gynecomastia
X-linked Genodermatoses
Usually, genes of one of the X chromosomes are inactivated in women (Lyonization). Thus X-linked genodermatoses are observed only in women, when the mutation is lethal in male offspring. This concerns the following syndromes: incontinentia pigmenti, focal dermal hypoplasia, Conradi-Hünermann-Happle syndrome, oralfacial- digital syndrome type 1 and MIDAS (microphthalmia, dermal aplasia and sclerocornea) syndrome, as well as in various X-linked non-lethal phenotypes, such as hypohidrotic ectodermal dysplasia of Christ-Siemens-Touraine, IFAP (ichthyosis follicularis-alopeciaphotophobia) syndrome and X-linked dyskeratosis congenita [1].
The two X chromosomes present in males with Klinefelter syndrome offer the potential that also males suffer from the syndromes mentioned above. Only a minor group, however, was observed until now and reported in the literature.
Incontinentia pigmenti (OMIM 308300, cytogenetic location Xq28) is caused by mutations in the NEMO gene in the IKK-gamma gene. The syndrome is an X-linked dominant disorder and is usually lethal prenatally in males. The 47, XXY karyotype is one of the mechanisms by which males may survive the effects of inheriting a lethal mutation. Seven cases of male IP and Klinefelter syndrome have been published in the literature until 2010 [2].
The clinical features of incontinentia pigmenti comprise linear skin lesions: early blistering with eosinophilia, eruption of hyperkeratotic lesions, hyper pigmentation along the lines of Blaschko, and dermal scarring (Figure 1).
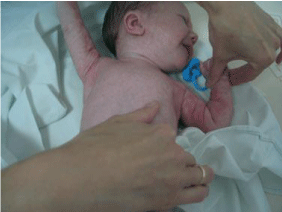
Figure 1: Incontinentia pigmenti: Papular, vesicular, and verrucous lesions
with an erythematous base arranged in lines on the face and scalp together
with watery and purulent vesicles on upper extremities (from [2], with
permission).
Focal dermal hypoplasia (OMIM 305600, cytogenetic locations: Xp11.23) is inherited as an X-linked dominant with in utero lethality in males. The features include atrophy and linear pigmentation of the skin, herniation of fat through the dermal defects, and multiple papillomas of the mucous membranes or skin (Figure 2). In addition, digital anomalies consist of syndactyly, polydactyly, camptodactyly, and absence deformities. Oral anomalies, in addition to lip papillomas, include hypoplastic teeth. Ocular anomalies have also been present in some cases.
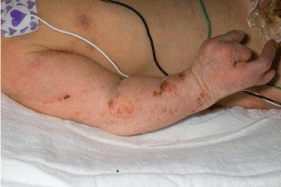
Figure 2: Focal dermal hypoplasia: Atrophic cutaneous and split hand
deformity (from [3], with permission).
A case report has been published [3], concerning a boy who survived due to the extra X chromosome.
Leg Ulcer
Patients with Klinefelter syndrome have a higher risk of the development of leg ulcers (Figure 3). The overall prevalence (for the adult population in the age range 18–79 years) of venous leg ulcers in western countries is estimated to be approximately 0.6% for healed and 0.1% for non-healed ulcers. The prevalence is strongly dependent on age [4].
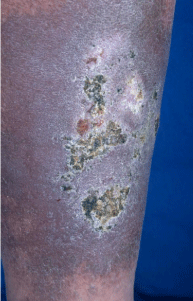
Figure 3: Leg ulcer as a consequence of preceding phlebothrombosis
localized in a skin area with massive hemosiderosis.
In younger patients, leg ulcers frequently are a consequence of post thrombotic syndrome, which is associated with an underlying hereditary haemostasis disorders. The most common hereditary disorders of haemostasis so far appear to be deficiencies of anti- thrombin III, protein C, and protein S, and activated protein C resistance.
The prevalence of leg ulcers in persons with Klinefelter syndrome is 6% to 13% 2, which is 3 to 30 times higher than that in the general population. Such leg ulcers are often refractory to treatment. Furthermore, determining that the ulcers are caused by Klinefelter syndrome often takes a long time [5]. Part of the predisposition is explained by their body height and obesity, since men with venous stasis are known to be significantly taller and more obese than agematched control subjects [4].
The serine protease inhibitor PAI-1 is the primary physiological inhibitor of tissue plasminogen activator and urokinase, which are activators of plasminogen and hence fibrinolysis. PAI-1 is elevated in a variety of thrombotic conditions, including deep venous thrombosis. The major sources for PAI-1 are adipocytes, and in obesity, expression of PAI-1 is dramatically up-regulated. Also in Klinefelter patients PAI-1 was found to be elevated, a causal relationship between elevated PAI-1 levels and androgen deficiency in Klinefelter syndrome, and androgen therapy normalizes both the low testosterone level and its associated high PAI-1 level [6]. Possible molecular mechanism explaining the association of Klinefelter syndrome and coagulation abnormalities are not reported in the literature.
Breast Cancer and Paget’s Disease
Klinefelter syndrome is a risk factor for male breast cancer (MBC). The prevalence is about 50 times higher than in normal males [7]. Other malignancies have not been observed with elevated frequency in Klinefelter patients, with the exception of lung cancer. Swerdlow et al. [8] compared the prevalence of cancer in a cohort of 3518 men who had been cytogenetically diagnosed with Klinefelter syndrome in Britain from 1959 through with that of men in the national population (Table 1)
Cancer site or type
No. of deaths
SMR (95% CI)
P
AER
Esophagus
5
1.2 (0.4 to 2.7)
0.84
1.4
Stomach
4
0.7 (0.2 to 1.9)
0.73
− 2.8
Colon and rectum
5
0.6 (0.2 to 1.3)
0.23
− 7.5
Pancreas
4
1.1 (0.3 to 2.9)
0.93
0.9
Lung
40
1.5 (1.0 to 2.0)
0.03
23.7
Melanoma
2
2.0 (0.2 to 7.2)
0.53
1.9
Breast
5
57.8 (18.8 to 135.0)
<.001
9.3
Prostate
0
Testis
0
Non-Hodgkin lymphoma
9
3.5 (1.6 to 6.6)
0.003
12.1
SMR: Standardized Mortality Ratio; CI: Confidence Interval; AER: Absolute Excess Risk per 100,000 Person-Years.
`
Table 1: Cancer mortality in 3518 patients with Klinefelter syndrome by selected cancer site or type [8].
Signs and symptoms of MBC are similar to those of FBC: a lump in the breast or a change in the nipple, such as discharge, retraction, or ulceration, which is reported in 27% of patients [9]. 85% of men present with painless subareolar mass. The lump is characteristically firm, irregular, and painless. In men with gynecomastia, the tumour is usually palpably distinct from the softer surrounding glandular tissue.
Paget’s disease is found in up to 5% of cases [10]. It appears as a scaling, erythematous, unshapely delineated lesion. It is accompanied by a palpable enlargement of the mammary gland (Figure 4). It has to be considered in particular, when skin changes of the male nipple and the alveolar area occur in association with a breast lump. Hayes et al. [11] summarized 43 published cases of Paget’s diseases in MBC on the basis of a Medline search. As rare variants of Paget’s disease, also pigmented lesions were described. Mushakis et al., [12] had collected 16 documented cases of Paget’s disease in patients with Klinefelter syndrome.
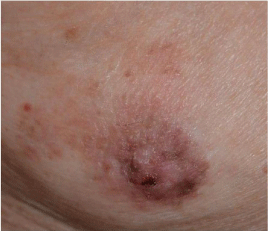
Figure 4: Paget’s disease of the nipple. Erosion and redness of the nipple
were noted, and lateral accretion. A 1.0-cm movable lump was identified in
the breast (from [21] via https://creativecommons.org/licenses/by/4.0/).
The cytogenesis of Paget disease is unclear. On the one hand, these cells represent equivalents of physiological Toker cells [13], which must particularly be considered if no ductal breast carcinoma is present. Toker cells possess a round, colorless nucleus and a paler cytoplasm than the surrounding keratinocytes. Toker cells are normally found at the tip of the nipple and in the skin of the areola; they are also found in supernumerary nipple-areola-complexes. They display immunoreactivity for cytokeratines 7, 8 and 18, but are negative for CEA, cerb-B2/HER2 and HPV-DNA. They originate from the clear cells of the lactiferous ducts [14]. The other possibility is that Paget cells originate from a beast cancer. The different immunoreactivity (Toker cells are negative for c-erb-B2/HER2, while breast carcinomas are consistently positive) can make differentiation possible.
Gynecomastia
Gynecomastia is an enlargement of the male breast. The male glandular tissue of the breast is estrogen-susceptible. Most authors address an “imbalance of androgen and estrogen action” as a pathogenic factor. The term gynecomastia is used in all types of increased breast volume and increased swelling of the male breast region, irrespective of the consistence and of the degree of swelling (Figure 5). The areola may be enlarged and stronger pigmented (Figure 6). This phenomenon reminds to the pigmentation of the female nipple and areola in hyperestrogenic states.
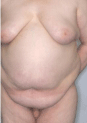
Figure 2: Gynecomastia in a patient with Klinefelter syndrome.
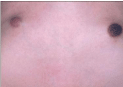
Figure 6: Enlargement and increased pigmentation of the areola in
gynecomastia.
Gynecomastia is a frequent feature in Klinefelter syndrome; exact figures, however, are scarce. Reviews describe a frequency of up to 50% in patients concerned [15]. About half of young men with gynecomastia suffer from Klinefelter syndrome [16].
Histopathologically, two types may be discriminated. Type I, the florid gynecomastia, is characterized by an increased number of ducts with irregular lumen, in some cases showing pseudo lobule formation. The epithelium may have more than three layers, sometimes with small papillae. The ducts may be surrounded by cuffs of connective tissue, which is well demarcated from the normal interlobular connective tissue. Type II, the quiescent gynecomastia, shows ducts with normal, unilayer epithelium, but irregular lumen and slight ectasia. No cuffs of connective tissue are seen. The stroma shows often hyalinization and no fibroblastic proliferation.
Treatment of gynecomastia may be performed pharmacologically, rediologically or surgically. The only drug appearing to be effective is the antiestrogenic compound tamoxifen. Mostly, it was used in adolescent gynecomastia. Tamoxifen was effective in up to 83% of patients. It appears to be well tolerated, even if long-term and placebo-controlled studies are lacking [17,18].
As prevention in hormonal treatment of prostatic cancer, radiotherapy is well proven [19]. Naturally, it is not applicable in adolescent gyncomastia.
The treatment of choice is surgery of the enlarged tissue is. There is a great variety of procedures [20], such as semicircular, intraareolar incision and resection of tissue, nipple transposition on single derma flap, free nipple graft after excision of redundant skin and breast tissue, transaxillary approach, liposuction. The latter is the most successful treatment from a functional and esthetic view.
References
- Happle R. X-chromosome inactivation: role in skin disease expression. Acta Paediatr Suppl. 2006; 95: 16-23.
- Buinauskaite E, Buinauskiene J, Kucinskiene V, Strazdiene D, Valiukeviciene S. Incontinentia pigmenti in a male infant with Klinefelter syndrome: a case report and review of the literature. Pediatr Dermatol. 2010; 27: 492-495.
- Alkindi S, Battin M, Aftimos S, Purvis D. Focal dermal hypoplasia due to a novel mutation in a boy with Klinefelter syndrome. Pediatr Dermatol. 2013; 30: 476-479.
- Gattringer C, Scheurecker C, Höpfl R, Müller H. Association between venous leg ulcers and sex chromosome anomalies in men. Acta Derm Venereol. 2010; 90: 612-615.
- Yabuno Y, Tosa M, Iwakiri I, Nomoto S, Kaneko M, Kuwahara K, et al. Refractory leg ulcers associated with Klinefelter syndrome. J Nippon Med Sch. 2015; 82: 64-67.
- Loskutoff DJ, Samad F. The adipocyte and hemostatic balance in obesity: studies of PAI-1. Arterioscler Thromb Vasc Biol 1998; 18: 1–6.
- Hultborn R, Hanson C, Köpf I, Verbiené I, Warnhammar E, Weimarck A. Prevalence of Klinefelter’s syndrome in male breast cancer patients. Anticancer Res. 1997; 17: 4293-4297.
- Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, Jacobs PA; UK Clinical Cytogenetics Group. Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J Natl Cancer Inst. 2005; 97: 1204- 1210.
- Agrawal A, Ayantunde AA, Rampaul R, Robertson JF. Male breast cancer: a review of clinical management. Breast Cancer Res Treat. 2007; 103: 11-21.
- Contractor KB, Kaur K, Rodrigues GS, Kulkarni DM, Singhal H. Male breast cancer: is the scenario changing. World J Surg Oncol. 2008; 6: 58.
- Hayes R, Cummings B, Miller RA, Guha AK. Male Paget’s disease of the breast. J Cutan Med Surg. 2000; 4: 208-212.
- Moshakis V, Fordyce MJ, Griffiths JD. Klinefelter’s syndrome associated with breast carcinoma and Paget’s disease of the nipple. Clin Oncol. 1983; 9: 257-261.
- Toker C. Clear cells of the nipple epidermis. Cancer. 1970; 25: 601-610.
- Di Tommaso L, Franchi G, Destro A, Broglia F, Minuti F, Rahal D, et al. Toker cells of the breast. Morphological and immunohistochemical characterization of 40 cases. Hum Pathol. 2008; 39: 1295-300.
- Nieschlag E. Klinefelter syndrome: the commonest form of hypogonadism, but often overlooked or untreated. Dtsch Arztebl Int. 2013; 110: 347-353.
- Yazici M, Sahin M, Bolu E, Gok DE, Taslipinar A, Tapan S, et al. Evaluation of breast enlargement in young males and factors associated with gynecomastia and pseudogynecomastia. Ir J Med Sci. 2010; 179: 575-583.
- Lapid O, van Wingerden JJ, Perlemuter L. Tamoxifen therapy for the management of pubertal gynecomastia: a systematic review. J Pediatr Endocrinol Metab. 2013; 26: 803-807.
- Wibowo E, Pollock PA, Hollis N, Wassersug RJ. Tamoxifen in men: a review of adverse events. Andrology. 2016; 4: 776-788.
- McLeod DG, Iversen P. Gynecomastia in patients with prostate cancer: a review of treatment options. Urology. 2000; 56: 713-720.
- Rohrich RJ, Ha RY, Kenkel JM, Adams WP Jr. Classification and management of gynecomastia: defining the role of ultrasound-assisted liposuction. Plast Reconstr Surg. 2003; 111: 909-923.
- Akita M, Kusunoki N, Nakajima T, Takase S, Maekawa Y, Kajimoto K, et al. Paget’s disease of the male breast: a case report. Surg Case Rep. 2015; 1: 103.
