Abstract
The abundant presence of saprophytes microorganisms in microbiological cultures has acquired interest in the study of infectious pathologies. The protective effect of coenzyme Q10 in mice testes infected with Staphylococcus epidermidis was evaluated. Sperm characteristics and testicular histology of male mice (NMRI) infected with S. epidermidis and infected and co-treated with coenzyme Q10 were studied. Sperm density, motility and morphology of epididymis, as well as testicular weight and histology were evaluated at 21 days post-treatment. Sperm abnormal forms and higher banana form and vacuolization in the seminiferous epithelium were observed in infected mice. The morphologic alterations were reduced after the treatment with coenzyme Q10 in co-trated group. Coenzyme Q10 may be an important therapeutic option in the reversal of sperm damage as potent antioxidant, in the restoration of reproductive tract epithelium and probably in infertility in mice infected with S. epidermidis.
Keywords: Coenzyme Q10; Staphylococcus epidermidis; Male accessory glands; Coagulase-negative Staphylococci
Introduction
Genital tract infections have been associated to infertility in many couples. The most studied microorganisms responsible among the causes of infectious infertility are Chamydia trachomatis, Mycoplasma hominis, Ureaplasma urealyticum, Neisseria gonorrhoeae and Treponema pallidum among others [1]. The impact of bacterial infection on seminal parameters are leukocyte production, with reduction of sperm density, normal forms and motility [2,3]. The aforementioned microorganisms have been associated with reduction of seminal characteristics; however, no specific changes have been defined in semen with high concentrations of Gram positive cocci. The frequency of Coagulase-negative Staphylococci (CoNS) in seminal samples has broad frequency ranges [4,5]. CoNS are considered saprophytes microorganisms of male urethra and are observed with higher frequency in semen of infertile men. A study showed associated the concentration of CoNS 104CFU/mL in semen with increased round cells (specially seminiferous epithelial cells instead leukocytes) and abnormal sperm forms (microcephalic) [6]. Another study showed that the same species of CoNS isolated in semen from infertile patients inoculated in testicles of rodents led to detachment of cells of the seminiferous epithelium and increase of compacted heads with smaller size and with lower acrosomal volume probably associated to oxidative stress [7].
A study demonstrated that species such as Enterococcus faecalis, Staphylococcus aureus and CoNS were observed in higher frequency in teratozoospermic men, but the finding have not been supported by statistical significance [3]. These observations make a point of debate that a saprophyte microorganism can cause damage to the male genital tract [8].
Staphylococcus epidermidis is the most frequent specie of CoNS, probably it has a beneficial role in the balance of the urethral microbiota of many epithelia to avoid the proliferation of harmful bacteria like S. aureus, so that, in special conditions S. epidermidis can behave as a nosocomial pathogenal [9]. In semen samples with S. epidermidis an increased apoptosis and ultrastructural changes have been observed [10]. The pathogenicity of S. epidermidis is due to the ability to produce adhesion factors, toxins, hemolysin, leucocidins and enterotoxins. Also, the formation of biofilms can inhibit the main mechanisms of defense in the host by means of production of protective surface polymers, exoenzymes and other cytolytic agents [9,11].
The mechanism that allows the conversion of a resident microbiota such as S. epidermidis to pathogen microorganism is difficult to explain [12]. A probable cellular mechanism in the host known as “extracellular ATP” (eATP) has been proposed, it may be an important physiological mediator of signaling associated to the conversion of opportunistic to pathogenic pro-inflammatory bacteria, producing a chronic infections [13]. The mechanism eATP has been associated with Reactive Oxygen Species (ROS) production and other extracellular signals involved in the pathophysiological response [14,15], so that, oxidative stress is a mechanism that can trigger the damage in the genital tract in presence of infections or inflammations [16]. ROS play an important role in male infertility; its levels of higher in semen of infertile men and the antioxidants could play an important role in protecting spermatozoa. Antioxidants have improved sperm density and normal forms in males with idiopathic oligoasthenoteratozoospermia [17].
The direct impact of microorganisms on the testis is not clearly established. In animal models the bacterial inoculation increases the leukocyte infiltration and reduces the spermatogenesis. These models have been applied to some bacteria whose pathogenicity ways are best known that CoNS infection [18-20].The protective role of an anti-oxidative agent as CoQ in immunity was demonstrated. A study showed that flies defective in CoQ biosynthetic were more susceptible to bacterial and fungal infections, in mammals the evidences aren´t enough [21]. This study evaluates the protective effect of coenzyme Q10 on the reproductive tract of mice infected with S. epidermidis.
Materials and Methods
Two groups of male mice (30 days old) were infected on testicular mediastinum with 0.1mL of S. epidermidis dilution; 6 of them were treated orally with coenzyme Q10 (group CoNS+Q), the other animals (n=6) were treated with placebo (group CoNS). The protocol followed the guidelines of the bioethics code of the University of Los Andes for laboratory animals [22]. The number of animals (n) was determined probabilistically with a 5% error α, 95% confidence level and the respective value p ≤ 0.05% [23]. Mice were weighed before and after treatment.
The inoculum was prepared from S. epidermidis obtained from a semen culture (106CFU/mL) of infertile men with normozoospermia. The bacterial inoculum was pre-incubated in Mueller Hinton broth (2.5mL) at 37°C for 6-8 hours, then it was diluted in physiological saline solution (0.85%) up to turbidity McFarland standard Nº 0.5 (1.5x108CFU/mL) [24].
The amount of 100mg contained into each capsule of coenzyme Q10 was diluted in 2.7mL of glycerin (100mg/3000μL). It was calculated on human dose (400mg/d) [25]. For an average of 28 grams in each mouse, a daily oral dose (160μg/5μL of the dilution) to 21 day was given, which were prepared 10 minutes prior to application. The group CoNS an oral dose of 5μL glycerol alone was given in the same period as in the other study group.
The animals were kept in polypropylene cages at an average temperature of 21±3°C for 3 weeks, and fed with commercial rat and sterilized water ad libitum in an environment of 12 hours of light and 12 hours of darkness. The animals were sacrificed with halothane [26,27]. The testes were removed, weighed and processed histologically into paraffin blocks, the sections were stained with hematoxylin eosin. The tubular compartments, the basal membrane of the tubules and the interstitial spaces were evaluated [28,29].
The epididymis were removed and placed in Eppendorf vials. Density and sperm morphology were measured from the right epididymis using the Dominguez morphological criteria with normal forms hooked, and abnormal forms: amorphous, banana-shaped and without a hook (Figure 1) [30] and sperm motility was evaluated from the tail of left epididymis [31]. The results were analyzed according to the multivariate, dichotomous, multifactorial design, through the Statistical Package for the Social Science (SPSS), version 17.0 to determine absolute frequencies.
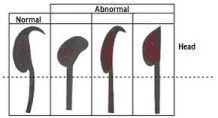
Figure 1: Sperm forms in mice (Authorized by Domínguez-Odio et al 2012).
Results
The body weight (grams) was similar between the groups CoNS and CoNS+Q after the treatment 32.2±1.7 vs 32.8±2.1, respectively. Testicular weight mean (left and right) did not show significant differences between the two groups.
Sperm density
No difference in sperm density was observed between the CoNS group (650±222x106 /mL) and CoNS + Q group (669±93x106 /mL) (p≥0.05). Increased non progressive motility was observed in the CoNS group, therefore, it was higher in the CoNS group (p≤0.05) (Figure 2).
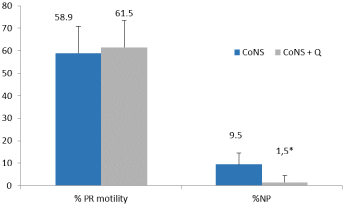
Figure 2: Percentage of sperm with progressive motility (PR) and nonprogressive
(NP).
Sperm morphology
The sperm forms banana, amorphous and without hook were higher in the CoNS group (p≤0.05) with respect to the CoNS+Q group (Figure 3).
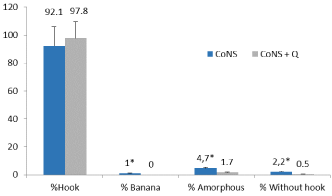
Figure 3: Percentage of sperm forms in group infected with S. epidermidis
(CoNS) and infected with S. epidermidis + coenzyme Q (CoNS+Q).
Histological analysis
Figure 4 shows transverse sections three weeks after the inoculation with S. epidermidis with mild hyalinization in the basement membrane, (4a): vacuolations were observed in some seminiferous tubules; (4b): and mild hypertrophy of the Leydig cells in sagittal cortex; (4c): In the cross section of the testes of the CoNS+Q group, germinal epithelium, interstitial space and the tubular lumen showed greater histological integrity. Some tubules were observed with moderate detachment of the germinal cells in their most apical area (4d).
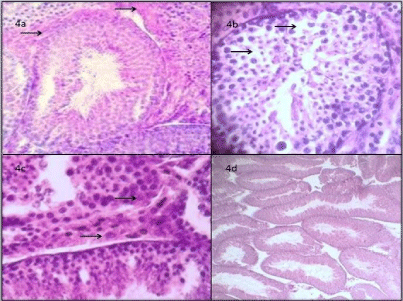
Figure 4: Group CoNS: testicular tissue 400X, transversal cortex with
seminiferous tubule with mild hyalinization of the basement membrane
(4a); some vacuolations on the epithelia of seminiferous tubules (4b); mild
hypertrophy of the Leydig cells in sagittal cortex (4c); Group CoNS+Q 100X
describes normal epithelium without alterations (4d).
Discussion
The animal model shows the negative effect of S. epidermidis on male genital tract. Sperm changes have been observed in abnormal forms in infected animals. A possible teratozoospermia-inducing factor has been found in humans, when globular forms are increased in the semen of infertile men and are associated with increased ROS morphologic characteristics of the sperm cell are the outcome of highly complex cellular modifications occurring during spermatogenesis [32]. The presence of abnormal spermatozoa in mice suggests specific structural abnormalities related to spermatozoa production and/or maturation associated to ROS over production [33,34].
Oral administration of coenzyme Q10 during months of treatment has allowed improve the morphology of spermatozoa in infertile men, especially by reduction of ROS levels that alter the permeability of the plasma membrane and head forms of the spermatozoa [35]. Treatment of subclinical infections and secretory failure of male accessory glands can improve sperm physiology to achieve spontaneous pregnancies [36].
The histological findings suggest the need to prescribe coenzyme Q10 to maintain the functional integrity of the seminiferous tubule before irreversible damage is generated, such as tubular hyalinization, with a significant alteration in the quality of spermatogenesis [37,38].
Conclusion
In summary, S. epidermidis causes microcytosis in sperm. The use of coenzyme Q10 may be the most favorable therapeutic option before the application of antimicrobial drugs, since the uncontrolled use of antibiotics can eliminate the resident microbiota on male genital tract or presenting complications associated with multiresistance to antibiotics.
References
- Solomon M, Henkel R. Semen culture and the assessment of genitourinary tract infections. Indian J Urol. 2017; 33: 188-193.
- La Vignera S, Condorelli RA, Vicari E, Salmeri M, Morgia G, Favilla V, et al. Microbiological investigation in male infertility: a practical overview. J Med Microbiol. 2014; 63: 1-14.
- Vilvanathan S, Kandasamy B, Jayachandran AL, Sathiyanarayanan S, Tanjore-Singaravelu V, Krishnamurthy V, et al. Bacteriospermia and its impact on basic semen parameters among infertile men. Interdiscip Perspect Infect Dis. 2016; 2614692.
- Leterrier M, Fréour T, Guillouzouic A, Juvin ME, Barriere P, Reynaud A, et al. Semen cultures analysis: retrospective study during a 6-year period and interest in the management of infertility. Eur J Clin Microbiol Infect Dis. 2011, 30: 401-406.
- Otto M. Staphylococcus epidermidis pathogenesis. Methods Mol Biol. 2014; 1106: 17-31.
- Lozano-Hernández R, Velasco J, Rodríguez M. Impact of Staphylococcus coagulase negative and other germs on sperm forms. Int J Med Res Health Sci. 2017; 6: 92-97.
- Lozano-Hernández R, Velasco J, Pacheco L, Sayago A, Peña J. Impacto de Staphylococcus epidermidis en el testículo murino. RVSVM. 2018.
- Yu W, Kim HK, Rauch S, Schneewind O, Missiakas D. Pathogenic conversion of coagulase-negative staphylococci. Microbes Infect. 2017; 19: 101-109.
- Galarzo S, Cano MA, Puerta J, Giraldo M, Mayorga J, Cadavid A, et al. Efecto de los factores solubles de Staphylococcus aureus, Staphylococcus capitis y Staphylococcus epidermidis sobre la funcionalidad espermática. Rev Chil Obstet Ginecol. 2015; 80: 316-323.
- Novick RP, Muir TW. Virulence gene regulation by peptides in staphylococci and other Gram-positive bacteria. Curr Opin Microbiol. 1999; 2: 40-45.
- Gdoura R, Kchaou W, Znazen A, Chakroun N, Fourati M, Ammar-Keskes L, et al. Screening for bacterial pathogens in semen samples from infertile men with and without leukocytospermia. Andrologia. 2008; 40: 209-218.
- Moretti E, Capitani S, Figura N, Pammolli A, Federico MG, Giannerini V, et al. The presence of bacteria species in semen and sperm quality. J Assist Reprod Genet. 2009; 26: 47-56.
- Von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002; 2: 677-685.
- Guerra AN, Gavala ML, Chung HS. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signal. 2007; 3: 39-51.
- Cogen AL, Nizet V, Gallo R. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008; 158: 442-455.
- Zimmermann H. Extracellular ATP and other nucleotides-ubiquitous triggers of intercellular messenger release. Purinergic Signal. 2016; 12: 25-57.
- Hewinson J, Mackenzie AB. P2X (7) receptor-mediated reactive oxygen and nitrogen species formation: from receptor to generators. Biochem Soc Trans. 2007; 35: 1168-1170.
- Sanui H, Yoshida S, Himeno K, Nomoto K. Delayed hypersensitivity to syngeneic testicular cells induced by intratesticular bacterial infection in guinea-pigs. Immunology. 1982; 46: 635-642.
- Baker DG. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev. 1998; 11: 231-266.
- Imamovic-Kumalic S, Pinter B. Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. Biomed Res Int. 2014; 2014: 426951.
- Cheng W, Song C, Anjum KM, Chen M, Li D, Zhou H, et al. Coenzyme Q plays opposing roles on bacteria/fungi and viruses in Drosophila innate immunity. Int J Immunogenet. 2011; 38: 331-337.
- Universidad de Los Andes. Bioterio. Comisión de Bioética. Aplicación para solicitar el aval por la comisión de bioética protocolos de investigación y docencia que involucran animales de laboratorio.
- Rojo A. Cálculo del tamaño muestral en procedimientos de experimentación con animales. Valoración de las incidencias. Animales de laboratorio. Verano. 2014.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. 2017.
- Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009; 182: 237- 248.
- Gallegos de Lerma G. Estudio morfológico de las alteraciones producidas por el etilmetanosulfonato, en células espermatogénicas de ratón. 1984. Tesis de maestría. Universidad Autónoma de Nuevo León, México.
- Álvarez Gómez de Segura I. Métodos de anestesia, analgesia y eutanasia- Capítulo14. Departamento de Cirugía Experimental. Hospital Universitario La Paz, Madrid.
- Kowalczuk CI, Saunders RD, Stapleton HR. Sperm count and sperm abnormality in male mice after exposure to 2.45GHz microwave radiation. Mutat Res. 1983; 122: 155-161.
- Itoh M, De-Rooij D, Takeuchi Y. Mode of inflammatory cell infiltration in testes of mice injected with syngeneic testicular germ cells without adjuvant. J Anat. 1995; 187: 671-679.
- Domínguez-Odio A, Puente-Zapata E, Pérez-Andrés IY, Salas-Pérez H. Solanum torvum Toxicidad sobre microorganismos y células espermáticas. Rev Med Inst Mex Seguro Soc. 2012; 50: 363-370.
- Boersma A, Olszanska O, Walter I, Rülicke T. Microsurgical and Percutaneous Epididymal Sperm Aspiration for Sperm Collection from Live Mice. J Am Assoc Lab Anim Sci. 2015; 54: 471-417.
- Han F, Liu C, Zhang L, Chen M, Zhou Y, Qin Y, et al. Globozoospermia and lack of acrosome formation in GM130-deficient mice. Cell Death Dis. 2017; 8: e2532.
- Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, Willard B, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013; 11: 85.
- Agarwal A, Tvrda E, Sharma R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. 2014; 12: 45.
- Shamsi MB, Venkatesh S, Kumar R, Gupta NP, Malhotra N, Singh N, et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J Biochem Biophys. 2010; 47: 38-43.
- Lozano R. Male accessory glands and sperm function. Intech Open. 2018; 101-116.
- Gual-Frau J, Abad C, Amengual MJ, Hannaoui N, Checa MA, Ribas-Maynou J, et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum Fertil (Camb). 2015; 18: 225-229.
- Tirabassi G, Vignini A, Tiano L, Buldreghini E, Brugè F, Silvestri S, et al. Protective effects of coenzyme Q10 and aspartic acid on oxidative stress and DNA damage in subjects affected by idiopathic asthenozoospermia. Endocrine. 2015; 49: 549-552.
