Case Report
Austin J Anesthesia and Analgesia. 2014;1(1): 1002.
Anesthesia Management of Endovascular Repair of Aortic Aneurysm
Hong Wang*
Department of Anesthesiology, Wayne State University, Detroit Medical Center, USA
*Corresponding author: *Corresponding author: Hong Wang, Department of Anesthesiology, Wayne State University, Detroit Medical Center 3990 John R, Detroit MI 48201, USA.
Received: December 02, 2013; Accepted: December 23, 2013; Published: December 31, 2013
A Case Study
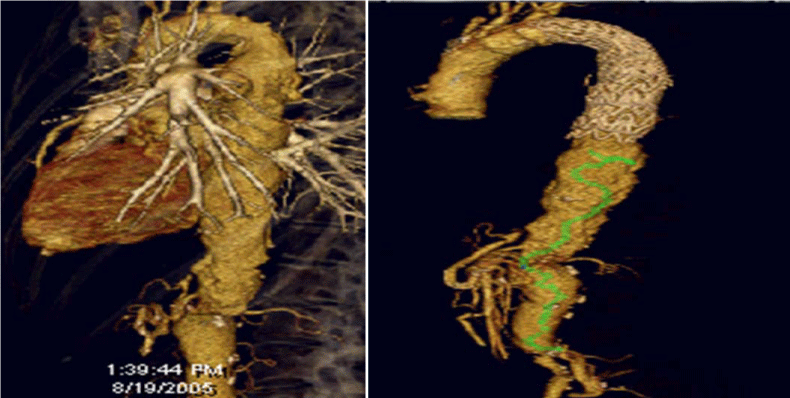
A 68-year-old female with tortuous aneurismal dilatation of the entire aorta with penetrating ulcers was scheduled for thoracoabdominal aneurysm endovascular repair. Her past medical history included previous abdominal aortic aneurysm (AAA) repair, coronary artery bypass, hypertension, Chronic Obstructive Pulmonary Disease (COPD), End Stage Renal Disease (ESRD), and active tobacco usage with 40 pack-year history. Due to the proximity of the stent deploy site and the origin of the aorta; adenosine was used to induce a 12-second asystole. A spinal drain catheter was placed and the pop-off pressure was 10 cm H2o to reduce the possibility of spinal cord injury. Transesophageal echocardiography (TEE) was used to guide the wires and estimate the endograft location. Due to the tortuous segmental dilation of the entire aorta, three different diameters of the aorta stents were deployed to fit the aorta. The patient was extubated at the end of the procedure and discharged from the hospital 5 days later. The following pictures show the aorta before and after the endovascular repair.
Introduction
Endovascular repair of aortic aneurysms has gained popularity in recent years as a less invasive and potentially safer alternative to the open procedures. During the open procedures, the extensive periaorta dissection, significant fluid shift, prolonged aortic occlusion, and potentially significant blood loss lead to the relatively high operative mortality in comparison to the endovascular approach. However, this advantage from the endovascular approach is offset in the long term by graft-related complications.
The Class I indication of endovascular repair of aorta is degenerative or traumatic aneurysms of the descending thoracic aorta exceeding 5.5 cm, saccular aneurysms, or postoperative pseudoaneurysms [1]. Candidates with multiple and significant comorbid medical conditions are often considered for endovascular repair.
Options/Therapy
Most aortic aneurysms can be treated by endovascular procedures if they meet the main technical criteria, including 20 mm of landing zone, distal vascular access, and limited tortuosity of the aorta.
Major adverse events related to endografts occur in 10-12% of patients in the initial 30-day perioperative period, with stroke rate between 2.5% to 8%, spinal cord ischemia in 1.5%, acute renal failure in 1.3%, and endoleaks in 10-20% of the patients [1]. Blood loss usually is not a serious concern. However, catastrophic bleeding does occur, but rarely. This could be due to the rupture of aneurysm or the retroperitoneal dissections to expose the external iliac artery.
Different approaches of this procedure have been developed. The retroperitoneal approach of abdominal aortic aneurysm (AAA) introduces the endografts through common iliac artery or aorta for patients with limited or no accessibility of the femoral artery [2]. Hybrid procedures for thoracic aortic aneurysm (TAA) and AAA have been developed for patients with aortic aneurysms involving major branches. These are exemplified by left carotid subclavian bypass [3], staged elephant trunk procedures [4], and aortic visceral debranching [5]. Although open surgical procedures are involved in these hybrid procedures, they are less invasive and involve less hemodynamic changes due to absence of aortic clamping. The open procedures can be staged or performed at the same time as endovascular procedures.
Most endograft patients have similar co-morbidities as open procedure patients. Some of them are not candidates for open procedures due to their coexisting conditions. The patients undergoing endovascular repair deserve the same extensive preoperative cardiac evaluation and intervention as recommended by the American College of Cardiology (ACC) and the American Heart Association (AHA) [6]. Whether the endovascular procedure should be classified as an intermediate or high-risk procedure is controversial due to the less hemodynamic change, fluid shift, and possibility of blood loss.
Figure 1: Thoracic and abdominal aorta before (1a) and after (1b) endovascular repair.
Invasive monitoring such as the CVP and arterial line during the procedure should take into consideration of the patient's co-morbidity, extensiveness of the aortic disease, and possibility of a conversion from the endovascular procedure to the open procedure. TEE has offered many advantages in monitoring and diagnosing ventricular functions and volume status. In addition, TEE can reveal pericardial effusion, presence of aortic regurgitation, the extent of a dissection, and the location of intimal tear. It also provides instantaneous views of the location of the guide wires and an estimation of the endograft location prior to deployment. TEE can also assist in diagnosing the endograft leakage and iatrogenic dissections.
Anesthesia techniques include general anesthesia, regional anesthesia, and local anesthesia. The choice of anesthesia depends on patient comorbidities, types and extensiveness of the aortic disease, the planned surgical intervention, and possibility of a conversion to open procedures. Scenarios will determine the necessity of general anesthesia, including but not limited to extensive dissection and exploration of the inguinal area to expose the femoral or iliac artery, complex surgeries, thoracic stent requiring TEE, likelihood of a conversion to open procedures, or induced rapid ventricular pacing or asystole to facilitate proximal graft deployment. We used general anesthesia for the reported case due to the extensiveness of the aortic disease, high possibility of a conversion to open procedures, requirement of TEE, and need of an induced asystole.
Intraoperative rupture is a rare but catastrophic event. A sudden, unexplained decrease in blood pressure should alert the possibility of this incident. The volume resuscitation should start immediately. General anesthesia should be initiated. Intraluminal balloon tamponade and subsequent deployment of the device beyond the area of rupture may effectively stop the bleeding although an emergent laparotomy is often necessary.
Several anesthesia techniques can be used during proximal graft deployment during endovascular TAA repair to prevent the device malposition. The techniques include induced hypotension, rapid ventricular pacing, and induced asystole. The induced hypotension can be achieved by sodium nitroprussida, nitroglycerine, short-acting beta-blockers, or calcium channel blockers [7]. Rapid ventricular pacing can be accomplished by either transesphogeal or transvenous pacemakers at the rates of 130 to 180 beats per minute to lower the systolic pressure to 50 to 60 mmHg. High-dose-adenosine-induced asystole has been used during the deployment of the graft although transit ST-segment depressions have been reported [8]. We successfully used this technique for the reported case. Adenosine 6 mg in escalating dose to 30 mg intravenously was able to provide the period of asystole for 25 seconds. The patient was able to resume regular cardiac rhythm without any myocardium ischemia changes. Other less used techniques include Valsalva maneuver and induction of transit ventricular fibrillation [9].
Spinal cord ischemia is a devastating complication after TAA or AAA above the celiac artery and can range up to 12% [10]. Compared to open procedures, endovascular repair has comparable cerebral vascular accidents and spinal cord ischemia incidents in the Medtronic Vascular Talent Thoracic Stent Graft System for the Treatment of Thoracic Aortic Aneurysms (VALOR) trial [11]. The incidence is correlated with the extent and concomitance of the disease [12] or previous abdominal aortic aneurysm repair [11]. During the stent placement, the intercostal arteries and arterial radicularis magna of Adamkewicz may be sacrificed. For spinal cord protection, cerebrospinal fluid (CSF) drainage is a Class I indication. The recommended set pressure is 8 to 10 cm H2O at which the CSF is allowed to drain. Class II indications include optimized spinal cord perfusion pressure and moderate systemic hypothermia [13].
Neurophysiological monitoring of the spinal cord such as somatosensory evoked potentials or motor evoked potentials are Class IIb indications. Details are discussed in the later sections.
Contrast induced renal failure is a serious complication for this procedure. Myoglobinuria resulted from reperfusion injury and aneurysm sac thrombosis with subsequent hemolysis can also contribute to the renal failure. The incidence of renal complications can be as high as 10%, especially in the patients with pre-existing conditions. Hydration and mannitol are Class IIb indications for the renal protection while lasix and dopamine are Class III indications according to Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease: Executive Summary [1]. Types of contrast can have different impact on renal function. Meta-analysis showed 50% reduction of incidents by low-osmolar agent in patients with preexisting renal insufficiency [14]. A recent report indicated that iso-osmolar further reduced the incidents [15]. Controversy exists on the impact of N-acetylcysteine on renal function [16]. Sodium bicarbonate infusion may offer some advantages although more studies are needed to reach conclusions [17]. The strategies to reduce the incidence of kidney injury should also include euvolemia, adequate perfusion pressure and cardiac output, limited contrast exposure, and sufficient time interval between procedures.
Clinical Evidence
Clinical outcomes of endovascular surgery in comparison to open procedures have been addressed in several studies. Significant reduction of operative mortality in endovascular surgeries (1.2% vs. 4.6%) was observed in a multi-center, randomized trial [18]. However, this early benefit was lost, partially due to fatal endograft rupture. Endovascular repair was associated with increased rates of graft-related complications and re-intervention, with increased cost [19]. In a study of Medicare beneficiaries undergoing aortic aneurysm repair between 2001 and 2004, more than 22,000 were propensity-score-matched. The survival advantage is more durable in aged patients [20].
Endovascular repair offers an option for patients who are ineligible for open repair. In an EVAR trial, within 404 patients with AAA > 5.5 cm in diameter who were considered to be physically ineligible for open repair, 197 patients were assigned to endovascular repair and the rest to no repair. The 30-day mortality was 7.3% for the repair group. The rate of rupture in the non-repair group was 12.4 per 100-person-year. Aneurysm-related mortality was lower in the endovascular-repair group. However, this advantage did not result in any benefit in terms of total mortality. 48% of patients who survived the endovascular repair had graft-related complications, and 27% required re-intervention within the first 6 years [21].
CSF drainage has been proved to reduce or reverse the incidence of spinal cord injury. In a study of 182 patients undergoing descending TAA repair, the incidence of spinal cord injury was reduced from 7% to 1% after employing the CSF drainage and augmentation of the distal perfusion pressure [22]. The randomized perspective study by Coselli further confirmed the benefit of CSF drainage. They randomized 145 patients with and without CSF drainage. CSF drainage resulted in 80% reduction in relative risk for postoperative neurologic deficits [23]. Similar results were also obtained in patients undergoing endovascular thoracic aortic repair [24]. In addition, CSF drainage in combination with augmentation of systolic blood pressure to > 90 mmHg can reverse the spinal cord injury if placed within 24 hours.
Uncertain Areas
Limited studies exist that compare outcomes from different anesthesia techniques. In an 8-year 164-center retrospective study, it was found that ICU admission was significantly lower for the local anesthesia group than the regional and general anesthesia groups. Hospital stay was significantly shorter in the local anesthesia group than the regional and general anesthesia groups. However, the patients appeared to be more selective in the local anesthesia group (less complex, less additional procedures) [25]. Old age and complexity of the surgery seem to be the main reason for the difference in general anesthesia outcomes. Obesity and necessity of iliac access may be a contraindication for local anesthesia.
Excess spinal drain may cause subdural hematoma. Dardik reviewed 230 patients who underwent thoracoabdominal aortic aneurysm (TAAA) repair with lumbar drain at the John Hopkins Hospital between 1992 and 2001. Eight (3.5%) patients had subdural hematomas. For seven of these eight patients, the drains were set to allow drainage for CSF pressure greater than 5 cm H2O. The mean amount (690 + 79 ml) of CSF removed from the patients who developed subdural hematoma was significantly greater than the amount removed from the others (359 + 24 ml) [26].
The efficacy of somatosensory (SSEP) and motor evoked potential (MEP) monitoring is undetermined. It is certainly beneficial in certain endovascular procedures such as long thoracic stent placement. MEP is more valuable in dealing with vulnerability of the posterior cord of spinal cord ischemia. However, its utility requires the withholding of muscle relaxant agent during the procedure. This makes management difficult, as does the requirement that only limited concentration of agents can be used during SSEP monitoring. SSEP loss is a delayed response of the spinal cord injury. Recently, transcranial motor evoked potentials (tcMEP) has been introduced into proximal descending aortic surgery [27]. The tcMEP was found to be sensitive in predicting the neurological outcome within 3 to 5 minutes following the ischemia assault [28].
Recently, near-infrared spectroscopy technology has been used to monitor brain and tissue oxygenation [29]. A preliminary report indicated that cerebral oximetry changes correlated with changes of spinal cord perfusion in a patient undergoing thoracic endovascular surgery [30].
Recommendations
Anesthesia management of endovascular aortic surgery should depend on the extent of the disease, surgical techniques, and the patient's comorbidity. Each of the treatment options discussed above must be individualized. An aortic rupture, although rare, should be recognized in a timely manner and effectively treated.
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive Summary. J Am Col Cardiol .2010; 55:e27-129. fending
- Derrow AE, Seeger JM, Dame DA, Carter RL, Ozaki CK, et al.The outcome in the United States after thoracoabdominal aortic aneurysm repair, renal artery bypass, and mesenteric revascularization. J Vasc Surg. 2001; 34:54-61.
- Derrow AE, Seeger JM, Dame DA, Carter RL, Ozaki CK, et al.The outcome in the United States after thoracoabdominal aortic aneurysm repair, renal artery bypass, and mesenteric revascularization. J Vasc Surg. 2001; 34:54-61.
- Woo EY, Carpenter JP, Jackson BM, Pochettino A, Bavaria JE, et al. Left subclavian artery coverage during thoracic endovascular aortic repair: a single-center experience. J Vasc Surg. 2008; 48: 555-560.
- Matsumura JS, Lee WA, Mitchell RS, Farber MA, Murad MH, et al. The Society for Vascular Surgery Practice Guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg. 2009; 50: 1155-1158.
- Greenberg RK, Haddad F, Svensson L, O'Neill S, Walker E, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation. 2005; 112: 2619-2626.
- Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, et al. Guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol. 2007; 50:e159-e241.
- Kahn RA, Moskowitz DM, Marin M, Hollier L. Anesthetic considerations for endovascular aortic repair. Mt Sinai J Med. 2002; 69: 57-67.
- Kahn RA, Moskowitz DM, Marin ML, Hollier LH, Parsons R, et al. Safety and efficacy of high-dose adenosine-induced asystole during endovascular AAA repair. J Endovasc There. 2000; 7: 292-296.
- Kahn RA, Marin ML, Hollier L, Parsons R, Griepp R. Induction of ventricular fibrillation to facilitate endovascular stent graft repair of thoracic aortic aneurysms. Anesthesiology. 1998; 88: 534-536.
- Morales JP, Taylor PR, Bell RE, Chan YC, Sabharwal T, et al. Neurological complications following endoluminal repair of thoracic aortic disease. Cardiovasc Intervent Radiol. 2007; 30: 833-839.
- Fairman RM, Criado F, Farber M, Kwolek C, Mehta M, et al.Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR trial. J Vasc Surg. 2008; 48: 546-554.
- Greenberg RK, Lu Q, Roselli EE, Svensson LG, Moon MC,et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008; 118: 808-817.
- Gravereaux EC, Faries PL, Burks JA, Latessa V, Spielvogel D, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg. 2001; 34: 997-1003.
- Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993; 188: 171-178.
- Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003; 348: 491-499.
- Pannu N, Manns B, Lee H, Tonelli M. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int. 2004; 65: 1366-1374.
- Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004; 291: 2328-2334.
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004; 351: 1607-1618.
- United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, et al. Endovascular versus open repair of abdominal aortic aneurysm. NEJM. 2010; 362:1863-1871.
- Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008; 358: 464-474.
- United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, et al. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med. 2010; 362:1872-1880.
- Estrera AL, Rubenstein FS, Miller CC 3rd, Huynh TT, Letsou GV, et al. Descending thoracic aortic aneurysm: surgical approach and treatment using the adjuncts cerebrospinal fluid drainage and distal aortic perfusion. Ann Thorac Surg. 2001; 72: 481-486.
- Coselli JS, LeMaire SA, Köksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: Results of a randomized clinical trial. J Vasc Surg. 2002; 35:631-639.
- Hnath JC, Mehta M, Taggert JB, Sternbach Y, Roddy SP, et al. Strategies to improve spinal cord ischemia in endovascular thoracic aortic repair: Outcomes of a prospective cerebrospinal fluid drainage protocol. J Vasc Surg. 2008; 48: 836-840.
- Ruppert V, Leurs LJ, Steckmeier B, Buth J, Umscheid T. Influence of anesthesia type on outcome after endovascular aortic aneurysm repair: an analysis based on EUROSTAR data. J Vasc Surg. 2006; 44: 16-21.
- Dardik A, Perler BA, Roseborough GS, Williams GM. Subdural hematoma after thoracoabdominal aortic aneurysm repair: an underreported complication of spinal fluid drainage? J Vasc Surg. 2002; 36: 47-50.
- Kakinohana M, Nakamura S, Fuchigami T, Sugahara K. Transcranial motor-evoked potentials monitoring can detect spinal cord ischemia more rapidly than spinal cord-evoked potentials monitoring during aortic occlusion in rats. Eur Spine J. 2007; 16: 787-793.
- de Haan P, Kalkman CJ. Spinal cord monitoring: somatosensory- and motor-evoked potentials. Anesthesiol Clin North America. 2001; 19: 923-945.
- Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 103 Suppl. 2009; 1: i3-13.
- Neal H. Badner, Nicolaou G, Clarke C, Thomas L.Forbes. Use of spinal near-infrared spectroscopy (NIRS) for monitoring spinal cord perfusion in endovascular repair of thoracoabdominal aneurysm. Proceedings, 13th Annual Outcomes Meeting, 2009, Accra Beach, Barbados, West Indies.2011; 25.