
Case Presentation
Austin J Anesthesia and Analgesia. 2017; 5(1): 1054.
Thoracic Epidural for Postoperative Pain Control after Video-Assisted Thoracoscopic Surgery (VATS) Lobectomy in a Patient with Mild Hemophilia A
Finzer B*, McSoley J and Lawrence J
Department of Anesthesiology, University of Cincinnati Medical Center, Cincinnati, Ohio, USA
*Corresponding author: Finzer B, Department of Anesthesiology, University of Cincinnati Medical Center, Cincinnati, Ohio, USA
Received: May 24, 2017; Accepted: June 14, 2017; Published: June 21, 2017
Abstract
The utilization of neuraxial techniques for analgesia in patients with clotting disorders has been reported for the management of pain from labor and delivery. Safe usage of these techniques relies on proper planning for the perioperative treatment of the clotting disorder, with emphasis on maintaining a normal factor level. We present a case of thoracic epidural placement and utilization for perioperative pain control for Video-Assisted Thoracoscopic Surgery (VATS) lobectomy in a patient with mild hemophilia A. The patient’s coagulation studies and factor levels were appropriately maintained prior to and throughout catheter indwelling time, and there were no signs or symptoms of any complication from epidural placement throughout this period.
Keywords: Thoracic epidural; Pain; Thoracoscopic surgery; Hemophilia A
Introduction
The utilization of neuraxial techniques, either spinal or epidural placement, in patients with clotting disorders has been reported for decades [1-5]. There are examples within the literature on the safe and effective use of neuraxial techniques in patients requiring epidural anesthesia/analgesia for labor and delivery [6,7]. These instances relied heavily on the coordinated effort and planning of the surgeon, anesthesiologist, and hematologist involved in the case. Proper planning for the perioperative treatment of the clotting disorder with emphasis on obtaining and maintaining normal coagulation studies and factor level(s) is crucial to patient safety [7]. The reports of epidural placement in patients with hemophilia A are lumbar epidural placements for lower limb surgery or for the management of labor pain, which involved a catheter dwell time of less than 24 hours [7]. There is a paucity of reports of thoracic epidural placement in non-obstetric patients and the use of indwelling catheters for more than 24 hours. Additionally, there is little literature that describes the longitudinal replacement protocols of Factor VIII in thoracic surgery patients to prevent perioperative bleeding. We present a case of thoracic epidural placement and its utilization for perioperative pain control for Video-Assisted Thoracoscopic Surgery (VATS) lobectomy in a patient with mild hemophilia A.
Consent for Publication
We were unable to obtain written or verbal consent for publication from the patient despite multiple attempts via telephone and written letter over a one-month period. The University of Cincinnati Institutional Review Board (IRB) determined that “this proposal does not meet the regulatory criteria for research involving human subjects. Ongoing IRB oversight is not required”.
Case Presentation
The patient is an elderly male with known mild hemophilia A who was found to have a lung nodule during evaluation for shortness of breath and cough. Subsequent Computed Tomography (CT) scan of the chest revealed a Left Lower Lobe (LLL) lung nodule measuring 23mm x 15mm x 19mm. The patient was referred to the thoracic surgeon for evaluation who then scheduled the patient for VATS lobectomy.
The patient was diagnosed with hemophilia A early in life after developing a sizable hematoma on the chest from a minor injury. His bleeding tendency has been mild, only requiring the use of factor replacement on two prior injuries (falls) and for three prior operations. He never experienced spontaneous hemorrhages or hemarthroses.
To develop a plan for the management of the patient’s hemophilia A; to prevent hemorrhage during surgery, facilitate the placement of invasive devices (arterial line and thoracic epidural), and reduce the risk of potentially devastating sequelae from epidural hematoma formation, the patient was referred to his hematologist and the anesthesiology preoperative clinic. The patient’s hematologist prescribed the following Factor VIII replacement protocol:
1. 4,600 units of factor VIII concentrate 1 hour prior to surgery.
2. 3,000 units of factor VIII concentrate every 8 hours thereafter.
3. Pre and post factor VIII activity checks with each dose to guide dose adjustment.
4. Goal: greater than 60% activity at nadir and greater than 100% activity after each dose.
The patient’s baseline laboratory data obtained two weeks prior to surgery revealed an International Normalized Ratio (INR) of 1.0, a Partial Thromboplastin Time (PTT) of 41.8, and factor VIII activity level of 19%. After the initial dose of recombinant factor VIII, the laboratory data revealed a PTT of 29.2 and factor VIII activity level of 182% normal (Figure 1 and 2).
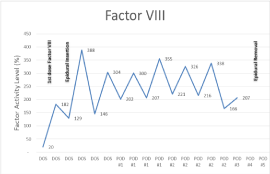
Figure 1: Factor VIII activity level during admission.
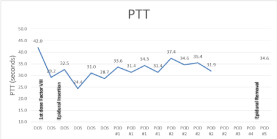
Figure 2: PTT (sec) during admission.
After confirmation of the normalization of the factor VIII activity level, an epidural was then placed using a 17-gauge Tuohy needle at the T6-7 inter space via a paramedian approach. Epidural insertion was completed with a trace amount of bleeding at the insertion site of the skin. No blood was seen in the needle or catheter. Aspiration of the catheter was negative for blood. A test dose of 3ml of 1.5% lidocaine with 1: 200,000 of epinephrine was given and found to be negative for signs of intravascular or intrathecal injection.
The patient underwent uncomplicated VATS lobectomy and was admitted to the surgical intensive care unit postoperatively. The hematology service assisted with the management of his hemophilia postoperatively using the above protocol. The patient’s dose of recombinant factor was decreased to 2000 units every 12 hours on postoperative day 3 by the hematology service because his factor VIII activity level was consistently greater than 150%. The patient’s factor VIII activity levels ranged from 129% to 388% throughout epidural catheter dwell time using this protocol and subsequent modification based upon the factor VIII activity levels.
The patient’s postoperative course was uncomplicated, and pain control was achieved using an epidural infusion of 0.125% bupivacaine and intravenous hydromorphone PCA (0.2mg, 6 minute lockout, no 4 hour limit). Pain control was assessed using the visual analog scale (VAS). The patient’s pain scores ranged 1 to 2 out of 10 on VAS while at rest and with deep breathing, but increased to 8 out of 10 while coughing on postoperative day 1. The epidural infusion was subsequently increased from 8ml/hr to 10ml/hr with improvement of patient’s pain on coughing to 6 out of 10 on VAS. The patient reported a tolerable level of pain control which permitted him to comfortably participate with the postoperative pulmonary hygiene therapy (ambulation, coughing, and incentive spirometry).
The chest tube was removed on postoperative day 3. The patient’s epidural catheter was removed on postoperative day 4 immediately following a dose of recombinant factor VIII. It was felt that the safest time for removal was immediately following a dose when factor levels are at a maximum.
The patient remained in hospital for 2 additional days following epidural catheter removal. He continued to receive recombinant factor VIII as prescribed by his hematologist during this time. The epidural insertion site was monitored after catheter removal and showed no signs of bleeding or hematoma formation. Follow up with the patient was performed via phone call 2 days after discharge. He had no signs of bleeding or hematoma at the epidural insertion site, and no signs or symptoms of epidural hematoma formation.
Discussion
Hemophilia A is a deficiency of clotting factor VIII, with severity based on the native factor VIII activity level. Patients with severe hemophilia A have a factor VIII activity that is less than 1%, those with moderate disease have activity between 1% and 4%, and those with mild disease have activity between 5% to 50% [6]. All coagulation disorders require careful coordination of care between surgeon, anesthesiologist, and hematologist in the perioperative period to minimize the risk of bleeding and reduce the need for blood transfusions.
A literature review of Pub Med revealed several case reports of lumbar epidural placement in patients with hemophilia A, as well as a literature review by Choi and Brull published in 2009. Their review of PubMed, MEDLINE, and EMBASE included 30 articles on neuraxial techniques in patients with clotting disorders published from January 1975 to October 2008. In their review, no thoracic epidural placement in a patient with hemophilia A was found [7]. However, lumbar epidural placement was deemed safe if correction of coagulation factor(s) to normal level(s) had occurred and was maintained [7].
Currently, there are no guidelines that sufficiently include all neuraxial techniques in patients with clotting disorders. The United Kingdom Haemophilia Centre Doctors’ Organization (UKHCDO) guidelines published in 2006 and the Australian Haemophilia Centre Directors’ Organization (AHCDO) position statement published in 2009 both state that epidural anesthesia for the management of labor pains is not contraindicated if a patient’s coagulation factors are maintained in the normal range during the duration of catheter placement [8,9]. The scarcity of reports, reviews, and guidelines makes setting goal values for perioperative coagulation studies difficult. Most guidelines emphasize the importance of maintaining a normal value of the deficient coagulation factor(s) as opposed to following traditional INR and PTT values [7-9] (Table 1). The minimum accepted factor values vary by report, but usually is stated to be 50% of normal [6-9]. A minimum factor VIII level of 60% of normal was chosen in this case in an effort to provide an added margin of safety.
Date/Time:
INR
PTT (s)
Factor VIII
Comments:
Baseline Values
1
42
20%
11/13/15 at 0631
1
29.2
182%
After first dose of Factor VIII prior to insertion of the epidural
11/13/15 at 1159
32.5
129%
Prior to Factor VIII
11/13/15 at 1230
24.4
388%
After Factor VIII
11/13/15 at 2015
31
146%
Prior to Factor VIII
11/13/15 at 2113
28.7
304%
After Factor VIII
11/14/15 at 0419
33.6
202%
Prior to Factor VIII
11/14/15 at 0514
31.4
300%
After Factor VIII
11/14/15 at 2016
34.3
207%
Prior to Factor VIII
11/14/15 at 2051
31.4
355%
After Factor VIII
11/15/15 at 0348
37.4
221%
Prior to Factor VIII
11/15/15 at 0434
34.6
326%
After Factor VIII
11/15/15 at 1122
35.4
216%
Prior to Factor VIII
11/15/15 at 1211
31.9
338%
Dosing changed to twice a day
11/15/15 at 2308
166%
Prior to Factor VIII
11/16/15 at 0001
207%
After Factor VIII
11/17/15 at 1124
Epidural removed
11/18/15 at 336
34.6
Prior to discharge
Table 1: PT/INR and Factor VIII activity levels at baseline, following initial dose, and during maintenance phase of therapy while epidural was in place.
The range of severity of Hemophilia A also makes standardized perioperative factor VIII replacement protocols difficult. In Choi and Brull’s review, no standard protocol was found or mentioned among the included articles, and treatment strategies ranged from intermittent dosing to constant infusions [6,7]. The theme of the recommendations and case reports are that factor levels were normal at the time of needle insertion and throughout the duration of catheter placement [7]. Since no reports of the utilization of thoracic epidural placement in patients with hemophilia A could be found, the coagulation goals from the aforementioned reports of lumbar epidural placement in women with hemophilia A were applied to this case. These reports, in addition to the standard risks, benefits, and alternatives to epidural placement, were discussed at length with the patient, who then elected to have a thoracic epidural placed for perioperative pain control. It was felt that all attempts at safe epidural placement be made in this patient given the nature of the procedure and known benefits of epidural analgesia in thoracic surgery.
This case is an example of the safe and effective use of thoracic epidural placement and utilization when factor VIII activity levels are maintained within the normal range prior to and throughout catheter placement for a patient with mild hemophilia A. The uncomplicated management of this patient also exhibits the need for coordination of care between multiple medical specialties to achieve a safe and successful outcome.
References
- Hack G, Hoffman P, Brackmann HH, Stoeckel H, Pichotka H. Regional anesthesia in haemophiliacs. Anasth Intensivther Not fall med. 1980; 15: 45- 51.
- Ahn DK, Jung WS, Lee JI. Hemophilia A in a senior patient: a case report of spinal epidural hematoma as first presentation. Asian Spine J. 2015; 9: 452-455.
- Bernhardt A, Bald C, Helfrich U, Haubelt H. Continuous lumbar epidural anesthesia: insertion a patient with unrecognized classical hemophilia A. Anesthetist. 2008; 57: 578-581.
- Faillace WJ, Warrier I, Canady AI. Paraplegia after lumbar puncture in an infant with previously undiagnosed hemophilia A. Treatment and perioperative considerations. Clin Pediatr (Phila). 1989; 28: 136-138.
- Heer JS, Enriquez EG, Carter AJ. Spinal epidural hematoma as first presentation of hemophilia A. J Emerg Med. 2008; 34: 159-162.
- Dhar, P, Abramovitz S, DiMichele D, Gibb CB, Gadalla F. Management of pregnancy in a patient with severe haemophilia A. Br J Anaesth. 2003; 91: 432-435.
- Choi S, Brull R. Neuraxial techniques in obstetric and non-obstetric patients with common bleeding diatheses. Anesthesia & Analgesia. 2009; 109: 648- 660.
- Lee CA, Chi C, Pavord SR, Bolton-Maggs PH, Pollard D, Hunchcliffe-Wood A, et al. Guidelines, The obstetric and gynecological management of women with inherited bleeding disorders - review with guidelines produced by a taskforce of UK Haemophilia Centre Doctor’s Organization. Haemophilia. 2006; 12: 301-336.
- Dunkley SM, Russell SJ, Rowell JA, Barnes CD, Baker RI, Sarson MI, et al. A consensus statement on the management of pregnancy and delivery in women who are carriers of or have bleeding disorders. MJA. 2009; 191: 460-463.