
Mini Review
Austin J Anesthesia and Analgesia. 2017; 5(3): 1063.
Ketamine: Variable Uses in Medicine
Sabia M¹*, Kudur V¹ and Reddy A²
¹Department of Anesthesiology, Cooper University Hospital, USA
²Department of Emergency Medicine, Cooper University Hospital, USA
*Corresponding author: Sabia M, Department of Anesthesiology, Cooper University Hospital, USA
Received: November 06, 2017; Accepted: November 28; 2017; Published: December 05, 2017
Abstract
Ketamine over the years has found its primary use in the setting of analgesia but recently studies have shown a spectrum of applications that range in a variety of specialties. The most profound utility is demonstrated in its application in acute and chronic pain, both of which has been addressed previously with opioids over the years. A more novel approach has been in the area of psychiatry where ketamine has found application in a variety of pathologies from depression to PTSD. The benefit is not only found in the efficacy of the intervention but the possible reduction of the side effect profile versus the standard of care. Further exploration of ketamine’s use in medicine will only build upon what we know and drive future application.
Keywords: Ketamine; Pain; Psychiatry; Depression; Acute; Chronic
Summary
Ketamine has a variety of uses in both acute and chronic pain, as well as sedation. In addition to its known effect on pain, recent studies are showing its use in psychiatric conditions as well. Despite ketamine’s benefits in clinical use, its precise mechanism still remains unknown. It is believed to act as NMDA and HCN1 receptors antagonist and possesses positive and negative modulatory role on analgesia and sedation. Chronic pain is often managed with ketamine for its hyper-glutamatergic properties in which the effects of the drug outlast the levels of the drug itself [1].
Introduction
Ketamine has been in use for nearly half a century as it produces a multitude of effects with dissociative anesthesia properties. The spectrum of effects includes hypnosis, anti-nociception, increased sympathetic activity, and maintenance of the airway [2].
Ketamine causes dependent blockade of the NMDA receptor which creates a blockade of the excitatory synaptic activity. This in turn creates a loss of responsiveness that allows it to aid in several clinical scenarios from acute and chronic pain to the most recent- as an antidepressant [1].
It has been shown that the effects of ketamine remain long after the drug has been excreted from the body, which makes it necessary to create a dose-response curve. This dose-response curve can be affected by a variety of factors. For instance, if the drug has a specific response in its molecular state in the lab, there must be a reason that same effect is not occurring in living organisms. One of the theories includes ketamine’s inability to penetrate the blood-brain barrier, or the presence of enzymes causing metabolism of the drug itself.
Molecular Actions of Ketamine
At concentrations within the clinical dose range, ketamine is now known to directly affect a wide range of cellular processes - including blockade of NMDA channels, nicotinic ACh channels, delta and muopioid and the nitric-oxide (NO)- cyclic guanosine-mono-phosphate (cGMP) system (Figure 1) [3].
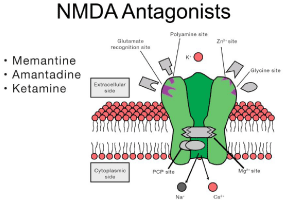
Figure 1: Ketamine disrupts multiple cellular processes on the extracellular
side of the NMDA receptor through itself and its metabolites. Interaction with
each subtype results in a different downstream result leading to a therapeutic
treatment.
Ketamine disrupts large number cellular processes, such as altered gene expression and protein regulation. NMDA control of calcium entry into the cell and the intracellular effects of calcium ions on protein and mitochondrial function can affect this [4].
Ketamine has been shown to result in suppression of immediate early gene expression at the site of mechanical injury. This alters the regulation of NMDA receptor phosphorylation and NMDA receptor mRNA expression that would result in a reduction in neuropathic pain [3].
NMDA action is influenced by competitive antagonists, open channel blocks, and non-competitive antagonists. Each compound has a different NMDA subtype which also results in a different spectrum of action [1]. For instance GLuN2b and GluN2a are both found within the brain however the GluN2B affects more of the limbic system, spinal cord and thalamus, while GluN2C is specific to the thalamus and cerebellum, and GluN2D to the brainstem, diencephalon, and spinal cord [2]. This allows ketamine to affect several parts of the brain through different channels and produce a variety of responses, each pertaining to different physiologic processes [1].
Pharmacology
Ketamine is metabolized into two major metabolites - norketamine and dehydronorketamine of the two, norketamine is the predominant metabolite and the non-competitive antagonist of the NMDA receptor, which contributes to ketamine analgesia. The halflife of ketamine varies between two and two and half hours (Figure 2) [5].
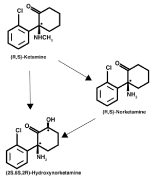
Figure 2: The breakdown of ketamine into two bioactive metabolites,
Norketamine and Hydroxynorketamine. Norketamine can be metabolized
down further into hydroxylnorketamine in the liver.
Administration routes for ketamine include IV, intramuscular, intranasal, epidural, subcutaneous, transdermal, intra-articular, sublingual and oral. Oral ketamine undergoes extensive first-pass liver metabolism resulting in a bioavailability of approximately 16%. The IM administration results in a bioavailability of approximately 93% [5].
When comparing IV and oral levels of ketamine, oral administration results in a higher level of drug than via IV administration. After IV administration, the plasma norketamine level rises but remains below the plasma ketamine level for about 2 hours. After administration, ketamine is rapidly metabolized into norketamine via cytochrome P450 enzymes in the liver, and norketamine is further metabolized into hydroxynorketamine. Ketamine and norketamine are both centrally acting NMDA receptor antagonists, while hydroxynorketamine has no pharmacological activity [6].
Once ketamine is metabolized to norketamine in the liver via cytochrome P450, the next step is to determine its effect compared to ketamine. Many studies measure the plasma concentration of ketamine and norketamine by using rifampicin, which is a CYP450 inducer. Using this method, the effectiveness of the metabolite and its actions are measured. When compared, it is said that norketamine is approximately 20-60% as potent as its pre-metabolized form, ketamine [6].
Ketamine Use in Pain
Until recently, IV ketamine was widely used as an acute analgesic. In recent years, several long terms trials have been developed about the uses of ketamine on chronic pain varying from days to weeks to months of pain relief. Although the studies show the positive effect of pain control, they have not completely addressed the safety concerns of the long term use. Ketamine has also been utilized in the treatment of patients with chronic refractory pain due to cancer and to reduce nonresponsive pain in patients with complex regional pain syndrome, and phantom limb pain [7].
Ketamine is structurally related to phencyclidine (PCP) and can be absorbed via IM, IV, oral, topical routes. It is metabolized by the hepatic microsomal enzymes specifically by cytochrome P450 enzymes including CYP3A4, CYP2B6, and CYP2C9. When ketamine is administered orally the first pass metabolism by CYP450 enzymes produces a variety of metabolites with the most active being norketamine [8]. Norketamine is metabolized to levels three times higher than the original ketamine agent but its drop in efficacy as a centrally acting NMDA receptor antagonist is notable. Norketamine acts as a non-competitive NMDA receptor antagonist which allows for a break in the activation of the neuronal pain cascade found in chronic pain. Norketamine is transformed into dehydronorketamine via hydroxylation with the help of CYP2B6 and CYP2A6. After further hydroxylation, these metabolites are able to be excreted in the bile and kidney [9].
Another metabolite of ketamine that is being explored for its efficacy in a clinical role is hydroxynorketamine. Hydroxynorketamine differs from its counterpart norketamine because of its indirect action on the NMDA receptor rather than direct. Hydroxynorketamine reduces the serum levels of D-serine, a coagonist of NMDA, and thus reducing NMDA excitation. In states of neuropathic pain, depression, chronic pain, and emotional stress D-serine may have the potential to be used as a biomarker for pre-and/or post response to ketamine [10].
Uses in Psychiatric Conditions
One of the newer uses for ketamine in addition to acute and chronic pain is for psychiatric conditions. These new uses for ketamine are still in the early stages and have yet to be established via long term meta-analysis. As of recent, there are no randomized, adequately controlled trials of repeated-dose ketamine for depression has been published. Evidence in support of repeated ketamine administration has been limited and ketamine therapy remains a highly experimental treatment approach. Due to similarities in brain network activity in depression and anxiety disorders, there is the possibility that ketamine might also be active in other refractory anxiety disorders. Ketamine may be a potential therapeutic alternative for patients with refractory generalized anxiety disorder/social anxiety disorder as well as post traumatic stress disorder [11-12].
One of the uses of ketamine for psychiatric conditions was described in the Trauma Interventions using Mindfulness Based Extinction and Reconsolidation (TIMBER) trial (2017) which analyzes the use of ketamine in PTSD patients along with specified psychotherapy. The goal of the trial was to establish the efficacy of combining dose related ketamine to patients with refractory trauma memories [12]. This works because ketamine is known to be a dissociative anesthetic agent and administration of ketamine (approx 0.5mg/kg body weight) augments the mindfulness state in the subject and causes a relaxed and dissociated mental state. This in turn allows the subject not to respond to the traumatic memories and accept them passively as they cross their mind. Throughout the periinfusion period, the arousal response is kept under control to avoid risk of retraumatization. This combined targeted approach integrates the strengths of both psychotherapy and ketamine and prevents restoration of traumatic experiences and the associated reactivity [13].
Another use for ketamine within psychiatry presents in the treatment of refractory bipolar disorder (BPD) as a rapid antidepressant rescue during acute depressive episodes. The basis for its efficacy lies in the presence of N-methyl-D-Aspartate (NMDA) receptor modulation in patients with symptomatic BPD. A randomized controlled trial conducted between 2006-2009 showed statistically significant improvements in depression within 40 minutes of receiving ketamine versus placebo. The intervention had this significant therapeutic result for 3 days with its peak benefit on day 2. Ketamine was administered by a single intravenous infusions dose 0.5mg/kg on 2 test days two weeks apart and the Montgomery As berg Depression Rating Scale was used to assess response. The evidence provided in this study opens the doors to ketamine as a rescue and potentially long term maintenance [14].
Dosing
Typically, 1-3 mg/kg of ketamine IV post surgery produce characteristic changes caused by ketamine. These changes include: normal pharyngeal-laryngeal reflexes, slightly enhanced skeletal muscle tone, and cardiovascular and respiratory stimulation, with minimal respiratory depression [6]. Depending on the dosage given, the clinical action of ketamine lasts between thirty minutes to two hours if administered intramuscularly, or four to six hours if administered orally [12]. Norketamine may also exhibit enantioselective pharmacological activity e.g. (S)-norketamine has an 8-fold higher affinity than (R)-norketamine in a rat cortical wedge separation. In a recent study, ketamine infusion was titrated for a 5 day infusion for 10-40mg/hour, and the plasma and urine concentrations of (S)-ketamine, (R)-ketamine, and their metabolites were obtained on day 3 of the infusion which revealed that the plasma concentrations of (R)-ketamine was greater than that of (S)-ketamine [15].
Adverse reactions
Ketamine causes many side effects including nausea/vomiting, hypertension, psychotropic effects, nightmares, and cognitive impairment. Several studies performed compare the norketamine plasma concentrations and how much analgesia is produced while monitoring side effects. Study analysis indicated that ketamine produced greater analgesia, psychotropic effects (drug high) and impairment of cognition than placebo medications [11].
Conclusion
Originally the uses for ketamine were limited to those related to analgesia. Over the past several years, it use has been extended into sedation, and more recently, psychiatric related uses. Its use in depression and anxiety is becoming more and more widely studied. Adequate trials have not yet been accomplished to determine the best dose range and long term side effects of ketamine with prolonged use in psychiatric patients.
Research does show that the role of ketamine pharmacology and metabolism are important factors in expanding the use for ketamine as many receptors and circuits for anxiety and depression are similar to those used in analgesia.
References
- Olofsen E, Noppers I, Niesters M, Kharasch E, Aarts L, Sarton E, and Dahan A. Estimation of the contribution of norketamine to ketamine-induced acute pain relief and neurocognitive impairment in healthy volunteers. Anesthesiology. 2012; 117: 353-364.
- Mion G and Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS neuroscience & therapeutics. 2013; 19: 370-380.
- Leung LY and Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. Journal of medicinal chemistry. 1986; 29: 2396-2399.
- Domino Edward F. Taming the ketamine tiger. 1965. The Journal of the American Society of Anesthesiologists. 2010; 113: 678-684.
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, and Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: A double-blind placebo controlled study. Pain. 2009; 147: 107-115.
- Cvrček Petr. Side Effects of Ketamine in the Long-Term Treatment of Neuropathic Pain. Pain Med. 2008; 9: 253-257.
- Grant IS, Nimmo WS, and Clements JA. Pharmacokinetics and analgesic effects of i.m. and oral ketamine. British Journal of Anaesthesia. 1981; 53: 805-810.
- Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer IW. Enantioselective pharmacokinetics of (R)-and (S)-ketamine after a 5-day infusion in patients with complex regional pain syndrome. Chirality. 2011; 23: 138-143.
- Morgan CJ, Curran HV. Ketamine use: A review. Addiction. 2012; 107: 27-38.
- Grant IS, Nimmo WS, and Clements JA. Pharmacokinetics and analgesic effects of i.m. and oral ketamine. British Journal of Anaesthesia. 1981; 53: 805-810.
- Kiefer RT, Rohr P, Ploppa A, Nohé B, Dieterich HJ, Grothusen J, et al. A Pilot Open Isomeric S (+)-Ketamine in Refractory CRPS Patients. Pain Med Pain Medicine. 2008; 9: 44-54.
- Kiefer RT, Rohr P, Ploppa A, Dieterich HJ, Grothusen J, Koffler S, et al. Efficacy of Ketamine in Anesthetic Dosage for the Treatment of Refractory Complex Regional Pain Syndrome: An Open-Label Phase II Study. Pain Medicine. 2008; 9: 1173-1201.
- Cvrček P. Side Effects of Ketamine in the Long-Term Treatment of Neuropathic Pain. Pain Med Pain Medicine. 2008; 9: 253-257.
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S. A Randomized Add-on Trial of an N-methyl-D-Aspartate Antagonist in Treatment-Resistant Bipolar Depression. Arch Gen Psychiatry. 2010; 67: 793-802.
- Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer I. Pharmacodynamic profiles of Ketamine (R-) and (S+) with five day inpatient infusion for the treatment of complex regional pain syndrome. Pain physician. 2010; 13: 379-387.