
Special Article - Anesthetic Management
Austin J Anesthesia and Analgesia. 2018; 6(2): 1071.
Anesthetic Management of Surgical Vascular Access for Hemodialysis
Nandate K¹*, Ava Alamdari¹ and Ramaiah R²
¹Associate Professor, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, USA
²Instructor, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, USA
*Corresponding author: Koichiro Nandate, Associate Professor Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, USA
Received: June 06, 2018; Accepted: July 04, 2018; Published: July 11, 2018
Keywords
Fistula; Renal Disease; Hyperkalemia; Anesthesia
Introduction
Patients suffering from end-stage renal disease (ESRD) have an adjusted all-cause mortality rate that is 6.4-7.8, fold higher than the general population. Additionally, chronic kidney disease (CKD) is an independent risk factor for predicting postoperative death and cardiac events [1]. Surgical techniques to establish hemodialysis access are common and increasing in frequency as more and more patients are diagnosed with advanced and end-stage renal disease.
The purpose of this review is to focus on the fundamentals of perioperative anesthetic management of a patient who scheduled for hemodialysis access procedure. This involves not just the choice of anesthesia method but also pre-anesthesia preparation, intraoperative and post-operative management.
Pre-Anesthesia Preparation
Pre-anesthesia clinic
Safe and effective anesthesia management starts with comprehensive preoperative evaluation. This is most efficiently done in a pre-anesthesia clinic where it is essential to identify the comorbidities that are common to the patients with chronic or end-stage renal disease, including coronary artery disease and hypertension. Once identified, measures should be taken to medically optimize these patients to minimize or eliminate the risk of surgery and anesthesia. Current guidelines recommend checking a baseline electrocardiogram (ECG) in those patients who have cardiovascular risk factors or documented cardiovascular disease [1]. Additionally, the patient should be instructed to schedule hemodialysis the day prior to the surgery, as well as counseled on what to do regarding their routine medications. Basic laboratory tests may also be helpful, including a complete blood count, metabolic panel and coagulation panel.
Same Day Evaluation Typically most procedures done to establish hemodialysis access are outpatient procedures with patients arriving 1-2 hours prior to the planned procedure. The pre-anesthesia evaluation in the pre-operative holding area is one of the most important phases in preparing the patient for the administration of anesthesia. The anesthesia team should review the pre-anesthesia evaluation that was done in the pre-anesthesia clinic and confirm that the patient’s general condition has not changed since that time. Intravenous access and blood pressure monitoring should be avoided in the arteriovenous (AV) access arm. Obtaining peripheral venous access may be difficult, and an ultrasound guided method may be helpful to identify an appropriate vein and secure access. In cases where a patient has an existing indwelling catheter, consideration can be taken to gain access however this is generally avoided due to fear of increased infectious complications .
Special Anesthetic Considerations: Chronic vs End-Stage Renal Disease
In those patients who have chronic renal disease but has not yet started hemodialysis, it is important to elicit history regarding the volume and regularity of urine production with special attention to those who report a recent drop in volume and/or frequency. This may indicate a recent worsening of their renal function, which may necessitate closer attention to potassium changes or fluid management during the procedure. For those patients already on hemodialysis, it is important to establish when the last time patient underwent hemodialysis was. Ideally, the patient should have hemodialysis 12 to 24 hours prior to the procedure to allow their physiologic status to completely or near completely return to normal at the time of anesthetic administration. Additionally, the regularity at which the patient has recently undergone dialysis is also important as a single session of dialysis may not normalize the patient that has missed more than one session, particularly with regard to fluid status. Similarly, it is important to ask if the patient felt comfortable during and tolerated the last hemodialysis session. If the patient felt uncomfortable during hemodialysis, or the session was terminated prematurely, or the patient skipped a regular session because of feeling ill, it may indicate other factors that must be taken into account. These factors, along with laboratory abnormalities may necessitate cancelling/rescheduling the procedure.
Preoperative laboratory data
Verification of certain laboratory data is critical to check on the day of the procedure as these patients are subject to day-to-day changes.
Potassium
The serum potassium levels in patients with chronic renal or endstage renal disease is typically elevated. It is essential to diagnose and treat hyperkalemia as it is potentially life threatening and therefore must not be neglected. There are no recommendations for absolute levels of pre-operative potassium levels that are considered safe. Therefore, the potassium level used to determine if the procedure can proceed or must be cancelled/rescheduled may vary among hospitals. It is worth noting that the serum potassium level is closely related with serum pH, thus if the patient is acidotic re-evaluation of serum potassium level must be considered after serum pH is corrected. In our institution, a potassium level higher than 6.0mmol/L prompts a discussion between the anesthesia and surgical teams regarding the need for urgent hemodialysis prior to the procedure. One additional consideration is that venous potassium levels can sometimes falsely be higher than arterial levels, and obtaining an arterial blood sample may be useful in confirming the correct true potassium level [2].
Occasionally, patients can have a lower preoperative potassium level (<3.5mmol/L). Hypokalemia is not as dangerous for patients as compared to hyperkalemia. Therefore, correction is required only if it is associated with frequent cardiac arrhythmias or with significant EKG changes such as QT prolongation. It is extremely difficult to correct hypokalemia in a patient with ESRD, and a nephrologist or cardiologist consultation maybe sought to avoid overcorrection with possible cardiac sequela.
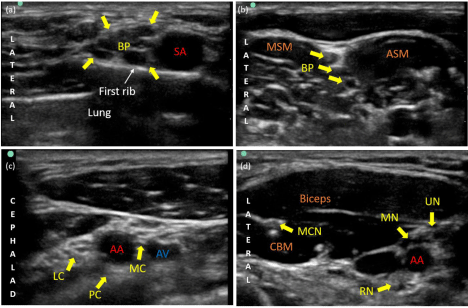
Figure 1: Ultrasound imaging of brachial plexus blocks. (a) Supraclavicular block, (b) interscalene block, (c) infraclavicular block and (d) axillary block.
Legends: BP: Brachial Plexus; SA: Subclavian Artery; MSM: Middle Scalene Muscle; ASM: Anterior Scalene Muscle; LC: Lateral Cord of the Brachial Plexus; PC:
Posterior Cord of the Brachial Plexus; MC: Medial Cord of the Brachial Plexus; AA: Axillary Artery; AV: Axillary Vein; MCN: Musculocutaneous Nerve; MN: Median
Nerve; UN: Ulnar Nerve; RN: Radial Nerve; CBM: Coracobrachialis Muscle.
Hemoglobin and Hematocrit
Most patients with chronic or end-stage renal disease are also affected by chronic anaemia due to lower erythropoietin activity, as well as the effect of uremic toxic metabolites on bone marrow. In general, anaemia does not need to be corrected because it is well tolerated by patients due to the gradual progression of anaemia. There are no definite guidelines as to the hematocrit level below which blood products should be transfused, but previous studies have reported increased intraoperative complications in patients with endstage renal disease and pre-operative Hct levels ranging from 20% to 26% [3]. Hemodialysis access surgery itself is usually not a procedure with significant surgical blood loss and therefore slightly more liberal criteria may be utilized for transfusion. Beyond specific objective criteria, such as hematocrit, transfusion should be considered if the patient is symptomatic or has significant comorbidities such as history of coronary artery disease and/or cerebrovascular disease. It should be noted that transfusion of blood products may increase the patient’s potassium level [4] as well as induce antibody formation which may decrease a patient’s chances of successful renal transplantation in the future [5].
Coagulation panel
Patients with chronic or end-stage renal disease have coagulopathy due to platelet dysfunction, decreased coagulation factors, and/or fragile capillary vessels. Additionally, they may have uncontrolled atrial fibrillation, cerebrovascular and/or peripheral vascular disease requiring chronic anticoagulation treatment, such as warfarin, or antiplatelet therapy, such as aspirin or clopidogrel. Patients are usually instructed to hold oral anticoagulants prior to the procedure but if this is not carried out there may be additional limitations to the surgery or could necessitate cancelling/rescheduling the procedure. In the case of a prolonged bleeding time or elevated INR a regional nerve block may be contraindicated due to risk of bleeding complications with hematoma formation and nerve compression.
Choice of Anesthesia
The fundamentals of anesthesia for vascular access surgery are essentially the same as those for other surgical procedures:
1. Keep the patients comfortable (reduce pain) along with immobility.
2. Optimize conditions for the surgeon.
3. Minimize risk of complications (e.g. perioperative cardiac events).
4. Optimize postoperative state - avoid prolonged sedation/ PONV and maintain adequate pain control.
The surgery for vascular access for hemodialysis in the operating room can essentially be categorized into two basic types of procedures: arteriovenous fistulas (AVF) and grafts (AVG). For AVF, a surgical anastomosis is created between an artery and a vein. There are two common sites in the upper extremity where AVF are created: between the radial artery and cephalic or basilic vein at the wrist and between the brachial artery and cephalic or basilic vein in the upper arm. For AVF created with the basilic vein, transposition of the vein is required which affects the method of anesthesia that is chosen. Alternatively, AVG are placed using prosthetic material between an artery and vein in the forearm or upper arm.
Anesthetic options available include local anesthetic (LA) infiltration around the operative site provided by the surgical team in combination with monitored anesthesia care and sedation (MAC) provided by the anesthesia team, regional anesthesia (RA) and general anesthesia (GA). Any of the three options are acceptable for both AVF and AVG. However, the patient’s medical co-morbidities, the anatomic location (wrist/forearm, antecubital fossa and upper arm), and the surgeon’s preference are to be considered when selecting the anesthesia method.
Choice of anesthetic options can be determined based on the anatomic location of surgical incision and LA with MAC can be considered suitable for the procedures performed at the wrist and the antecubital fossa. RA is a viable option for procedures performed at the antecubital fossa and distal upper arm. GA or RA with an interscalene block can be considered when procedures involve the proximal upper arm and for AVG and transpositions which require tunneling.
Local anesthesia
Infiltration of LA in the surgical field by the surgeon provides stable anesthesia with minimal to no hemodynamic and respiratory changes, and is therefore often used in patients who have severe comorbidities such as recent myocardial infarction, severe coronary artery disease and chronic obstructive pulmonary disease. The specific LA selected depends on the surgeon’s preference, but many surgeons prefer 1% lidocaine as the onset is faster compared to other LA. The maximum dose of lidocaine usage has been reported to be 5mg/kg. In some patients LA alone is not well tolerated, as they may experience agitation or anxiety during the procedure. However, this can be overcome by sedation/analgesia provided by the anesthesia team. An additional limitation to LA is the lack of an effect on the flow characteristics of the artery, in contrast to regional and general anesthesia. Previous reports have revealed that the use of a brachial plexus block with a supraclavicular approach provided dilatation of both the veins and arteries of the ipsilateral extremity immediately following the block, reduced the incidence of arterial spasm during and after the surgery, and significantly decreased the rate of immediate AVF failure postoperatively when compared to those that were performed with LA [6-8].
Regional anesthesia
RA of the upper extremity is primarily achieved through a brachial plexus block. RA offers many advantages over other anesthetic methods, including intraoperative hemodynamic stability and good postoperative analgesia. There is also evidence that it improves vascular flow via regional sympathectomy, although evidence of improved graft survival is lacking [6-8]. There are several ways to perform a brachial plexus block, including interscalene, supraclavicular or infraclavicular and axillary approaches. Complications of RA include infection, hematoma, local anesthetic toxicity and nerve injury. There are also complications that are specific to each approach, such as total spinal anesthesia, Horner syndrome, hemidiaphragmatic paralysis and pneumothorax/hemothorax during interscalene or supraclavicular blocks. The interscalene or supraclavicular block should be avoided in patients who are unable to withstand up to 30% reduction in pulmonary function resulting from ipsilateral phrenic nerve block. Although at this time there is little published data, the use of ultrasound guided nerve blocks certainly appear to have made these blocks easy and decrease the incidence of some of these complications [9]. It is important to note that following a brachial plexus block supplementation with LA by the surgeon may be required and LA dose calculations are be kept in mind avoiding local anesthetic toxicity.
General Anesthesia
Almost all patients with CKD and ESRD have multiple comorbid risk factors for GA due to the nature of the conditions that led to the renal insufficiency. Previous reports indicate that approximately 25% of the patients who undergo renal replacement therapy have ischemic heart disease, 10% have cerebrovascular disease and 12% have peripheral vascular disease [10]. For these reasons GA is avoided when possible but this may not always be feasible. This is often the case for patients with a history of psychological disorders or those who need more complicated procedures, such as an upper arm transposition or AVG, which may not be amenable to RA. Modes of GA delivery include endotracheal tube (GETA) and laryngeal mask (LMA). There are some advantages of GETA over LMA. It provides a more secure airway and PaCO2 is more easily controlled, resulting in minimal aspiration risk and avoiding the alkalosis that can contribute to decreasing the potassium level rapidly. However, usage of LMA does not require muscle relaxants, which can delay emergence from GA at the conclusion of the case.
During anesthesia induction, hemodynamics should be maintained with titrating doses of inductions agents such as propofol, muscle relaxants and prompt use of narcotics. However, blood pressure typically drops significantly after induction due to lower vascular compliance and/or lower cardiac reserve function. In these cases, vasoactive medications, such as ephedrine and phenylephrine intravenously as a bolus or a continuous infusion, should be utilized to keep the perfusion pressure adequate. For the pain control during the surgery, the use of LA by the surgeon can reduce the intraoperative use of inhalational anesthetics and narcotics.
Intraoperative Management
Potassium level
With the administration of any type of anesthesia, potassium may rise to a critical level suddenly. Therefore, close attention should be paid to ECG changes. Even minor changes of the QRS complex or the height of the T wave should prompt the immediate collection of a blood sample to check the potassium level. If elevated, immediate treatment to decrease the potassium should be initiated.
Treatment for hyperkalemia involves immediate administration of calcium (10ml of 10% calcium chloride). A bolus dose of insulin (5-10 units while checking serum glucose simultaneously) should be followed by a continuous infusion of D10W with 5-10 units of regular insulin per 25-50g of glucose. After this, sodium bicarbonate (50 to 100 mEq) and furosemide (if the patient still can make urine) should be administered. Other methods to decrease the potassium level includes increasing the respiration rate (if the patient is under a GA). Frequent checks of the potassium level should be performed until it is normalized.
Heparin
The surgeon will request heparin prior to clamping the artery. It is important to verify the dose of heparin, and flush the lines to confirm the administration.
Oxygenation status
In cases undertaken with LA, RA or LMA and where a patient is requiring high doses of sedatives, it can become difficult to maintain the patency of the airway. This can place the patient at risk of hypoxia. In this situation there should be a pause in the procedure so that an adjunctive airway device can be placed using an oral or nasal airway, or securing the airway with conversion to GETA. Inserting an airway instrument alone is sometimes enough to stimulate the patient causing them to move suddenly which is one reason that the procedure should be paused during the intervention.
Postoperative Anesthesia Care
Anesthesia management does not discontinue at the end of the surgical procedure but when the patient is discharged from the post anesthesia care unit. It should be noted that potassium level may increase suddenly even in the perioperative period. Therefore, the recovery nurse should pay close attention for ECG changes and possibly check the serum potassium. Occasionally the timing of the next HD session may need to be advanced, especially for the patient that missed their regular HD session prior to the procedure. The criteria are varied between hospitals, but consulting a nephrologist should be considered and common for any institute.
References
- National institute for clinical excellence (NICE). Preoperative tests. Clinical Guidelines. 2003.
- Wongyingsinn M and Suksuriyayothin S. Use of rapid ABG analyzer in measurement of potassium concentration: Does it agree with venous potassium concentration? J med Assoc Thai. 2009; 92; 925-929.
- Kllerman PS. Preoperative care of the renal patient. Arch Intern Med. 1994; 154: 1674-1688.
- Brenowitz JB, Williams CD and Edwards WS. Major surgery in patients with chronic renal failure. Surgey. 1977; 134: 765-769.
- Poli F, Scalamogna M, Cardillo M, Porta E, Sirchia G. An algorithm for cadaver kidney allocation based on a multivariate analysis of factors impacting on cadaver kidney graft survival and function. Transpl Int. 2000; 13: 259-262.
- Malinzak EB and Gan TJ. Regional anesthesia for vascular access surgery. Anesth Analg. 2009; 109; 976-980.
- Reynolds TS, Kim KM, Dukkipati R, Nguyen TH, JulkaL, Kakazu C, et al. Pre-operative regional block anesthesia enhances operative strategy for arterilvenous fistula creation. J Vasc Access. 2010; 12; 336-340.
- Shemesh D, Raikhinstein Y, Orkin D, Goldin I and Olsha O. Anesthesia for vascular access surgery. 2014; 15; 38-44.
- Neal JM. Ultrasound-guided regional anesthesia and patient safety: An evidence-based analysis. Reg Anesth Pain Med. 2010; 35: 59-67.
- Craig RG, Hunter JM. Recent developments in the perioperative management of adult patients with chronic kidney disease. Br J Anaesth. 2008; 101: 296- 310.