
Research Article
Austin J Anesthesia and Analgesia. 2018; 6(2): 1073.
Impact of Ketorolac on Opioid Consumption after Knee Arthroscopy
Wilson SH¹*, Slone H², Furse CM1, Epperson TI1, and Wolf BJ3
¹Department of Anesthesia and Perioperative Medicine, Medical University of South Carolina, USA
²Department of Orthopedics, Medical University of South Carolina, Charleston, SC, USA
³Department of Public Health Sciences, Medical University of South Carolina, Charleston, SC, USA
*Corresponding author: Wilson SH, Department of Anesthesia and Perioperative Medicine, Medical University of South Carolina, USA
Received: October 15, 2018; Accepted: November 01, 2018; Published: November 08, 2018
Abstract
Purpose: The objective of this study was to examine postoperative opioid consumption in outpatients undergoing knee arthroscopy after a single dose of intravenous ketorolac.
Methods: Patients ages 18-65 years old, weighing over 50kg and scheduled for knee arthroscopy were randomized to one of the four groups of preoperative ketorolac (0mg, 7.5mg, 15mg, 30mg). The primary outcome measured was postoperative opioid consumption. Secondary outcomes included visual analog scale pain scores, patient satisfaction scores, side effects and total postoperative anesthesia care unit time. Equivalency between ketorolac groups in opioid reduction relative to placebo was evaluated for each dose pair (7.5 vs. 15mg, 7.5 vs. 30mg, and 15 vs. 30mg). Linear regression models were used to examine associations between ketorolac dose with postoperative length of stay and patient satisfaction. A linear mixed model was used to evalaute the association between ketorolac dose and pain scores over time.
Results: A total of 112 patients with comparable patient and procedural characteristics were enrolled. Equivalency in opioid reduction relative to placebo was not demonstrated between any examined ketorlac doses (7.5 vs. 15mg, P = 0.167; 7.5 vs. 30mg, P = 0.451; 15 vs. 30mg, P = 0.515). Compared to placebo, all ketorlac doses decreased postoperative pain scores (global P=0.012). Patient satisfaction and postoperative duration did not vary with ketorolac dose.
Conclusions: Although all ketorolac doses decreased PACU pain scores, equivalency in PACU opioid reduction between ketorolac doses was not demonstrated.
Keywords: Arthroscopy; Ketorolac; Analgesics, Non-Narcotic; Pain, Postoperative
Abbreviations
IQR: Interquartile Range; IV: Intravenous; ME: Morphine Equivalents; NSAID: Non-Steroidal Anti-Inflammatory Drug; OR: Operating Room; PACU: Post Anesthesia Care Unit; SD: Standard Deviation; SE: Standard Error; TOST: Two One-Sided Test; VAS: Visual Analog Scale
Introduction
Ketorolac is a parental non-steroidal anti-inflammatory drug (NSAID) with potent analgesic activity [1]. A nonselective inhibitor of cyclooxygenase, it has reversible effects on platelets and a side effect profile similar to ibuprofen. In a variety of orthopediatic procedures, ketorolac has been shown to provide analgesia similar to opioids, but with less respiratory depression, somnolence, urinary retention, confusion, constipation, and ileus [2-5].
Since the side effects of ketorolac such as gastrointestinal upset and surgical site bleeding are dose related, utilizing a lower dose may be more desirable [6]. High dose ketorolac (>120mg/day) has been associated with impairment in spinal fusion healing while low or normal dose ketorolac (<120mg/day) has not been associated with an increased risk of non-union [7]. Further, in patients receiving intravenous (IV) ketorolac every six hours after spine surgery, the 7.5mg and 15mg doses were as effective as the 30mg dose in decreasing postoperative pain and opioid consumption [8].
Ketorolac is often administered to patients undergoing ambulatory knee arthroscopy. However, because prior studies have focused on a 30mg dose [4-5, 9-11], the optimal dose for ambulatory orthopedic surgery has not been determined. Since ketorolac side effects are dose related [6], a lower dose of ketorolac (7.5mg and 15mg) would be an attractive option if it provided effective analgesia. Our primary aim was to examine postoperative opioid consumption in patients undergoing knee arthroscopy after a single dose of IV ketorolac. We hypothesized that the reduction in opioid consumption in the Post Anesthesia Care Unit (PACU) would be equivalent in the three ketorolac groups compared with placebo.
Methods
Following institutional IRB approval and written informed consent, patients undergoing elective, ambulatory knee arthroscopy were enrolled in this single center, double blinded, randomized, prospective, placebo controlled study. Inclusion criteria included ASA physical status scores I-III, ages 18-65 years old, weight over 50kg and scheduled for minor knee arthroscopy (meniscus surgery, chondral debridement, loose body removal, synovectomy) using 2-3 ports under general anesthesia with a laryngeal mask airway. Exclusion criteria included inability to provide informed consent, contraindications to non-steriodal anti-inflammatory drugs (NSAIDs; allergy, chronic kidney disease, gastric or peptic ulcers, gastritis, gastrointestinal bleeding, severe volume depletion, presence of cerebrovascular bleeding or high risk of bleeding), allergy to medications used in the study protocol (propofol, fentanyl, hydromorphone), taking NSAIDs the morning of surgery, chronic painful conditions (opioid use over the last six months), emergency surgery, ligament/tendon repair or reconstruction, pregnancy, lactating, provider refusal or patient refusal. Non-English speaking patients were also excluded due to the limited availability of interpreter services.
Patient care and data collection
Patient care was standardized throughout the perioperative period. After informed consent, subjects were consectively assigned a three digit enrollment number (001-112). Subject randomization was based on a computer-generated list created by the statistician prior to study initiation and kept in the pharmacy. All participants, surgeons and anesthesiologists were blinded to the randomized ketorolac dose (0mg, 7.5mg, 15mg, or 30mg); only the pharmacist preparing the medication was aware of dose. Study medication was delivered in a syringe labeled “ketorolac study drug” with the subject’s enrollment number and diluted with saline to a total volume of 3ml. Saline was used for placebo (0mg) patients. The study drug was administered immediately prior to transport to the operating room (OR). All participants received a standardized general anesthetic (IV propofol, fentanyl, and ondansetron with sevoflurane). A long acting local anesthetic (ropivacaine or bupivacaine, 30ml) was injected in the portals and intra-articular space by the surgeon prior to anesthetic emergence. After anesthetic emergence, patients were transported to the PACU. Postopertive pain management was also standardized. In the PACU, an RN collected visual analog scale (VAS) pain scores on arrival and discharge. All VAS measurements throughout the study were made by having subjects mark their level of pain on a 10cm line. The first VAS measurement on PACU arrival was obtained once the patient was able to do so and prior to any pain medication administration in PACU. Hydromorphone (0.2mg IV every 5 minutes) was administered for a pain score >3 until pain relief was achieved. Hydromorphone was held for deep sedation, (>2 on Ramsay sedation scale) or in the presence of apnea, oxygen desaturation, or respiratory rate less than 10 breaths per minute. Side effects were recorded and medications were available for treatment, including shivering (meperidine 6.25mg IV), nausea (promethazine 6.25mg IV) or itching (diphenhydramine 12.5mg IV).
Outcomes
The primary outcome was PACU opioid consumption. All opioids were recorded as they were administered and converted to IV morphine equivalents (ME) after data collection concluded (10mg morphine IV calculated equal to fentanyl 0.1 mg IV, hydromorphone 1.5mg IV, meperidine 75mg IV, or hydrocodone 30mg PO) [12-13].
Secondary outcomes included VAS pain at PACU arrival and discharge, occurence of side effects and overall patient satisfaction. This data was collected throughout the perioperative period. Preoperative data collection included patient demographics and two baseline VAS pain measurements (worst pain in the past week and pain in holding on the day of surgery). These VAS measurements served to gauge the severity of the patient’s pre-existing pain and familiarize subjects with VAS score reporting. Intraoperative data collection included medications administered, total operative time (procedure start to end), and total OR time (patient in to out of OR). Postoperative data measurement included medications administered, VAS scores (at PACU admission and discharge), PACU time and side effects (nausea, vomiting, itching, respiratory distress, increased sedation or prolonged somnolence, intractable pain or other). Data was entered into a database by one investigator and rechecked by a second for quality assurance.
Statistical analysis
Preliminary data collection indicated that ketorolac 30mg would result in a 50% (±20) reduction in PACU opioid consumption compared to placebo. Therefore, to examine the reduction in opiate reduction between groups compared with placebo, a priori power analysis found that 28 patients per group were required (total n=112) to achieve 80% power at a significance level of P=0.0167 (Bonferroni adjusted for 3 pairwise comparisons) A reduction of 30% or greater in other ketorolace groups would indicate equivalence to the 30mg ketorolac dose group.
The primary outcome was postoperative PACU opioid consumption. The primary hypothesis was that the reduction in opioid consumption compared to the placebo group would be equivalent in the three ketorolac dose groups. Differences between the each ketorolac group and the placebo group in postoperative opioid consumption were estimated from linear contrasts from a regression model of opioid consumption. Equivalency between ketorolac groups in opioid reduction relative to placebo was evaluated using a two onesided test (TOST) approach for each dose pair (7.5 vs. 15mg, 7.5 vs. 30mg, and 15 vs. 30mg).
Secondary outcomes were evaluated for associations with ketorolac dose. VAS pain scores over time were evaluated using a linear mixed model including fixed effects for ketorolac dose and time (PACU arrival or discharge) and a random subject effect to account for repeated pain measures on the same subject. Patient satisfaction and PACU length of stay were evaluated using a linear regression approach. Due to the limited number of participants experiencing side effects, no formal hypothesis testing was conducted to evaluate the association with ketorolac dose. For all regression models, model assumptions were checked graphically and transformations were considered when necessary. Additionally, for models where the global P-value for ketorolac dose was significant at P ≤ 0.05, pairwise comparisons between each active treatment dose with placebo were evaluated using linear contrasts and Dunnett’s adjustment for multiple comparisons. All analyses were conducted in SAS v. 9.4 (SAS Institute, Cary, NC).
Results
The study enrolled 112 patients with 28 patients randomized to each group (Figure 1) between April 2012 and July 2016. Enrollment ceased with study completion. Patient and operative characteristics were balanced by randomized ketorolac dose assignment with the majority undergoing partial meniscectomy (Table 1). Preoperative VAS pain measurements also did not differ by ketorolac group.
0mg
7.5mg
15mg
30mg
(n = 28)
(n = 28)
(n = 28)
(n = 28)
Gender (Male)
14 (50.0)
20 (71.4)
13 (46.4)
16 (25.4)
Race (White)
21 (24.7)
22 (25.9)
23 (27.1)
19 (22.4)
Age (Years)
49.4 (11.9)
44.2 (12.7)
44.7 (14.2)
44.4 (13.0)
Weight (kg)
88.0 (19.2)
96.9 (24.0)
89.8 (19.7)
95.0 (21.5)
BMI (kg/m2)
29.8 (5.18)
31.1 (7.51)
29.7 (6.05)
31.1 (7.05)
ASA Score
1
13 (46.4)
9 (32.1)
13 (46.4)
8 (28.6)
2
11 (39.3)
11 (39.3)
12 (42.9)
15 (53.6)
3
4 (14.3)
8 (28.6)
3 (10.7)
5 (17.9)
Pain in Week Prior
5.0 (5.6)
5.5 (4.6)
5.2 (4.7)
5.8 (6.0)
Pain Day of Surgery
0.4 (1.8)
0.47 (1.5)
0.6 (2.0)
0.6 (3.0)
Total OR Time (min)∫
60.1 (18.4)
61.5 (16.5)
55.8 (18.9)
59.4 (16.2)
Operative Time (in)∫
32.4 (15.9)
33.8 (14.8)
31.0 (18.7)
33.9 (15.5)
Fentanyl (µg)*
100 (50)
100 (50)
100 (72.5)
100 (50)
Categorical variables are reported as n (%)¥ and continuous variables are reported as mean (SD)∫ or median (IQR)*.
Abbreviations: SD: Standard Deviation; IQR: Interquartile Range; VAS: Visual Analog Scale.
Total Operative time was measured as time between incision and closure.
Table 1: Patient and operative characteristics by randomized ketorolac dose.
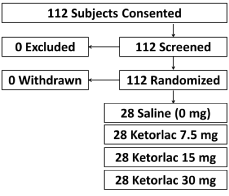
Figure 1: CONSORT flow diagram detailing patient enrollment and
randomization.
Postoperative opioid conmsumption
The primary outcome was postoperative PACU opioid consumption (Figure 2). Mean PACU opioid consumption by ketorolac dose is presented in Table 2. Equivalency in opioid reduction relative to placebo was not demonstrated between any examined ketorlac doses (7.5 vs. 15mg, P = 0.167; 7.5 vs. 30mg, P = 0.451; 15 vs. 30mg, P = 0.515). Although the groups receiving 7.5 and 15mg of ketorolac had similar mean opioid consumption, equivalency was not demonstrated due to a wide range in opioid consumption in these groups.
0mg
7.5mg
15mg
30mg
P
Opioid Consumption (mg)
7.7 (0.86)
6.3 (0.86)
6.5 (0.86)
5.0 (0.86)
0.182
VAS (cm)
PACU Arrival
4.6 (0.40)
3.3 (0.39)
3.3 (0.39)
3.0 (0.39)
0.012
PACU Discharge
3.2 (0.40)
2.0 (0.39)
1.9 (0.39)
1.7 (0.39)
0.012
Patient Satisfaction (cm)
8.9 (0.29)
9.0 (0.28)
9.5 (0.28)
9.7 (0.28)
0.123
PACU Time (minutes)
75.4 (5.6)
69.9 (5.5)
68.9 (5.5)
73.9 (5.5)
0.808
Values are presented as mean (SE). All P-values are for the global test for ketorolac dose group.
Opioid consumption is presented in intravenous morphine equilavents.
Abbreviations: PACU: Postoperative Anesthesia Care Unit; SE: Standard Error; VAS: Visual Analog Scale.
PACU time was measured as time between patient admission and discharge to PACU.
Table 2: Primary and secondary outcomes by ketorolac dose groups.
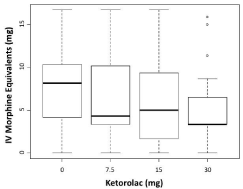
Figure 2: Postoperative opioid consumption by ketorolac dose group. Boxes
represent median and 25th-75th percentiles. Whiskers represent 1.5 times the
interquartile range (IQR). Open circles represent observed values outside 1.5
times the IQR.
Secondary outcomes
Additional secondary ouctomes are presented in Table 2. Ketorlac administration was associated with decreased PACU VAS pain scores (Global P=0.012). Specifically, patients in the placebo group reported signficantly higher pain than patients receiving ketorolac (0 vs. 7.5 mg, P=0.038; 0 vs. 15mg, P=0.029; 0 vs. 30mg, P=0.007) (Figure 3). Patient satisfaction and PACU duration were not associated with ketorolac dose (Global P=0.123 and P=0.808, respectively). Incidence of side effects by ketorolac dose group are presented in Table 3. However, given the limited number of participants reporting side effects no statistical evalautions were done.
0mg
7.5mg
15mg
30mg
(n = 28)
(n = 28)
(n = 28)
(n = 28)
Any Side Effects
9 (32.1)
4 (14.3)
3 (10.7)
7 (30.4)
Itching
0 (0.0)
0 (0.0)
0 (0.0)
2 (7.1)
Nausea
4 (14.3)
4 (14.3)
2 (7.1)
3 (10.7)
Somnolence
3 (10.7)
0 (0.00)
0 (0.00)
0 (0.00)
Pain Difficult to Control
3 (10.7)
0 (0.00)
1 (3.6)
1 (3.6)
Other
3 (10.7)
0 (0.00)
0 (0.00)
3 (10.7)
Data are reported as n (%).
Table 3: Side effect by randomized ketorolac dose.
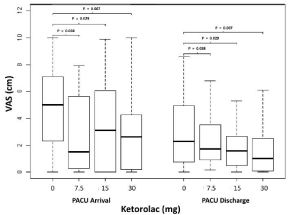
Figure 3: Visual analog scale (VAS) pain scores (cm) on arrival and discharge
from the postoperative anesthesia care unit (PACU) by ketorolac dose group.
Boxes represent median and 25th-75th percentiles. Whiskers represent 1.5
times the interquartile range.
Discussion
This study examined various doses of ketorolac on PACU opioid consumption following knee arthroscopy in an ambulatory surgical setting and found all of ketorolac groups to reduce postoperative opioid consumption compared with placebo. However, despite similar mean opioid consumption among ketorolac groups, a wide range in postoperative opioid consumption was noted in the 7.5 and 15 mg ketoroalc groups. This resulted in lack of equivalency between ketorolac groups when examining opiate reduction compared to plaebo.
While previous studies have supported using ketorolac for the treatment and prevention of postoperative pain following orthopedic surgery, the majority of prior studies have examined does of 30mg or greater with adminstration in single or repeated doses [2-5,9-11,14,15] The impact of ketorolac doses less than 30mg has not been studied as frequently. Our results found that all three examined doses (7.5, 15, and 30mg) decreased postoperative pain realtive to placebo. Zhou et al reported similar findings in a double blind, placebo controlled study in patients undergoing hip or knee arthroplasty where patients were randomized to placebo, propacetamol (2g) or ketorolac (15 or 30mg) [14]. Both ketorolac doses and paracetamol were noted to provide improved analgesia, measured as decreased pain scores, compared with placebo; although, the 30mg ketorolac dose had a faster anlagesic onset. In another randomized, double blinded study, spine patients received postoperative IV saline or ketorolac (5, 7.5, 10, 12.5, 15 or 30mg) every 6 hours [8]. They found similar 24 hour morphine consumption and pain scores in patients receiving ketorolac doses of 7.5mg or greater. More recently, Duttchen et al did not find 30mg of IV ketorolac superior to 15mg following lumbar decrompression spine surgery [16]. Both of these last two studies differ from ours in that they both tested for superiority between ketorolac doses.
Notably, our results also demonstrated that all doses of ketorolac examined decreased postoperative pain. Specifically, compared to placebo decreases in our PACU VAS scores ranged from 1.3-1.6cm on arrival to 1.2-1.5cm on discharge compared with placebo. While these decreases may seem minor, recent publications have found the minical clinically important difference for VAS after orthopedic surgery to be only 1.4cm [17]. Past studies have also noted ketorolac to decrease postoperative pain scores without reducing opioid consumption [10,14,18]. Ng et al found ketorolac (30mg IV) to lower PACU pain scores compared parecoxib (40mg IV), but did not find a difference in the number of patients requiring rescue analgesics [18]. Likewise, in a double blind, placebo controlled study in patients undergoing lower extremity arthroplasty, ketorolac improved pain scores compared with placebo, but did not reduce rescue analgesic administration [14]. Similarly, a large meta-analysis of 782 patients concluded that while ketorolac was an effective adjunct in a multimodal pain regimen and provided significant relief with rescue administration to patients in moderate to severe pain, it did not offer a significant opioid-sparing effect with pre-emptive ketorolac (30mg IV) adminstration [19]. Conversely, a randomized, placebo controlled study in patients undergoing minor othropedic surgery found that opioid consumption and pain scores were similar if ketorolac (30mg IV) was administered pre-emptively or for analgesic rescue [10].
Limitations
Opioid consumption was lower with greater range in opioid consumption within a dose group. Lower than expected opioid consumption in the placebo group resulted in a less than 50% reduction in the 30mg ketoroloac group compared with placebo. Due to a wide range in the opioid consumption in the groups receiving 7.5 and 15mg of ketorolac, equivalency was not demonstrated despite similar mean opioid consumption, due to a wide range in opioid consumption in these groups. Pain was only assessed in the immediate postoperative period. Since ketorolac has a short half-life (5 hours), an effect on late pain control would not be expected, but this could be examined in future studies. Given the limited number of side effects reported in our study population, we are underpowered to detect differences in side effects between groups.
Conclusions
Ketorolac should be considered as part of a multimodal analgesic regimen for patients undergoing knee arthroscopy. Although all ketorolac doses decreased PACU pain scores, equivalency in PACU opioid reduction between ketorolac doses was not demonstrated.
References
- Gillis JC, Brogden RN. Ketorolac: A reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997; 53: 139-188.
- Barber FA, Gladu DE. Comparison of oral ketorolac and hydrocodone for pain relief after anterior cruciate ligament reconstruction. Arthroscopy. 1998; 14: 605-612.
- Etches RC, Warriner CB, Badner N, et al. Continuous intravenous administration of ketorolac reduces pain and morphine consumption after total hip or knee arthroplasty. Anesth Analg. 1995; 81: 1175-1180.
- DeAndrade JR, Maslanka M, Reines HD, et al. Ketorolac versus meperidine for pain relief after orthopaedic surgery. Clin Orthop Relat Res. 1996; 325: 301-312.
- Alexander R, El-Moalem HE, Gan TJ. Comparison of the morphine-sparing effects of diclofenac sodium and ketorolac tromethamine after major orthopedic surgery. J Clin Anesth. 2002; 14: 187-192.
- Strom BL, Berlin JA, Kinman JL, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding: A postmarketing surveillance study. JAMA. 1996; 275: 376-382.
- Li Q, Zhang Z, Cai Z. High-dose ketorolac affects adult spinal fusion: A metaanalysis of the effect of perioperative nonsteroidal anti-inflammatory drugs on spinal fusion. Spine. 2011; 36: 461-468.
- Reuben SS, Connelly NR, Lurie S, Klatt M, Gibson CS. Dose-response of ketorolac as an adjunct to patient-controlled analgesia morphine patients after spinal fusion surgery. Anesth Analg. 1998; 87: 98-102.
- Kim JT, Sherman O, Cuff G, Leibovits A, Wajda M, Bekker AY. A double-blind prospective comparison of rofecoxib vs ketorolac in reducing postoperative pain after arthroscopic knee surgery. J Clin Anesth. 2005; 17: 439-443.
- Morrow BC, Bunting H, Milligan KR. A comparison of diclofenac and ketorolac for postoperative analgesia following day-case arthroscopy of the knee joint. Anaesthesia. 1993; 48: 585-587.
- Vanlersberghe C, Lauwers MH, Camu F. Preoperative ketorolac administration has no preemptive analgesic effect for minor orthopaedic surgery. Acta Anaesthesiol Scand. 1996; 40: 948-952.
- Harvey NR, Wolf BJ, Bolin ED, Wilson SH. Comparison of analgesia with lumbar epidurals and lumbar plexus nerve blocks in patients receiving multimodal analgesics following primary total hip arthroplasty: A retrospective analysis. Int Orthop. 2017; 41: 2229-2235.
- Wilson SH, Wolf BJ, Algendy AA, Sealy C, Demos HA, McSwain JR. Comparison of Lumbar Epidurals and Lumbar Plexus Nerve Blocks for Analgesia Following Primary Total Hip Arthroplasty: A Retrospective Analysis. J Arthroplasty. 2017; 32: 635-640.
- Zhou TJ, Tang J, White PF. Propacetamol versus ketorolac for treatment of acute postoperative pain after total hip or knee replacement. Anesth Analg. 2001; 92: 1569-1575.
- Cassinelli EH, Dean CL, Garcia RM, Furey CG, Bohlman HH. Ketorolac use for postoperative pain management following lumbar decompression surgery: A prospective, randomized, double-blinded, placebo-controlled trial. Spine. 2008; 33: 1313-1317.
- Duttchen KM, Lo A, Walker A, et al. Intraoperative ketorolac dose of 15mg versus the standard 30mg on early postoperative pain after spine surgery: A randomized, blinded, non-inferiority trial. J Clin Anesth. 2017; 41: 11-15.
- Tashjian RZ, Hung M, Keener JD, et al. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. J Shoulder Elbow Surg. 2017; 26: 144-148.
- Ng A, Temple A, Smith G, Emembolu J. Early analgesic effects of parecoxib versus ketorolac following laparoscopic sterilization: A randomized controlled trial. Br J Anaesth. 2004; 92: 846-849.
- De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: A meta-analysis of randomized trials. Anesth Analg. 2012; 114: 424-433.