
Case Presentation
Austin J Anesthesia and Analgesia. 2019; 7(1): 1076.
Persistent Central Neuropathic Pain Caused by Intramedullary Hemorrhage from Spinal Dural Arteriovenous Fistula: A Case Report and Literature Review
Iampreechakul P¹*, Lertbutsayanukul P², Siriwimonmas S³, Jittapiromsak P4, Tantivatana J5 and Niruthisard S6
¹Department of Neurosurgery, Prasat Neurological Institute, Bangkok, Thailand
²Department of Neuroradiology, Prasat Neurological Institute, Bangkok, Thailand
³Department of Radiology, Bumrungrad International Hospital, Bangkok, Thailand
4Division of Neurosurgery, Department of Surgery, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
5Department of Radiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
6Department of Anesthesiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
*Corresponding author: Iampreechakul P, Department of Neurological Surgery, Prasat Neurological Institute, 312 Rachawithi Road, Khwaeng Thung Phaya Thai, Bangkok 10400,Thailand, E-mail: bangruad@ hotmail.com
Received: January 07, 2019; Accepted: January 25, 2019; Published: February 01, 2019
Abstract
We describe a patient with persistent central neuropathic pain caused by intramedullary hemorrhage from spinal dural arteriovenous fistula (SDAVF). A 34-year-old woman suffered from sudden severe electric-like pain and paresthesia at the left anterior and posterior chest wall below nipple line, corresponding with T6 dermatome, without muscle weakness or bowel/ bladder dysfunction involving. Magnetic resonance imaging (MRI) revealed intramedullary hemorrhage extending from the level of lower T5 to upper T7 of the left side of the spinal cord with abnormal intradural flow voids along left posterolateral cord surface from the level of T6 to T11. Spinal angiography demonstrated SDAVF, fed by radiculomeningeal branches from the left T5 and T6 intercostal arteries with drainage into ascending and descending prominent and tortuous perimedullary draining veins. There was a venous varix, probably causing hematomyelia. The left T6 intercostal artery not only gave rise the branch to the fistula, but also anterior spinal artery. Therefore, endovascular treatment with liquid embolic material was contraindication for this patient. Due to intractable at-level neuropathic pain, she underwent thoracic laminectomy with microsurgical obliteration of the fistula and dorsal root entry zone lesioning in the same session. The previous chest pain preoperatively was totally relieved for a few days after surgery. Unfortunately, the neuropathic pain gradually returned with stabbing, cramping, and itching sensation. The painaggravating factors were premenstrual period, stress, mechanical pressure, and fear of untreatable pain. The pain- relieving factors were warm bath and gentle rub. Intractable neuropathic pain was treated with multi-drug therapy, including opioid, tricyclic antidepressant, and antiepileptic drugs. At 2 years after operation, the pain was controlled in acceptable level with pain score of 2/10. Follow-up spinal angiography and MRI confirmed complete obliteration of the fistula and disappearance of blood components in spinal cord without spinal cord atrophy. From the literature, the authors found another 5 patients suffering from intramedullary hemorrhage caused by SDAVFs. However, there was no persistent neuropathic pain in these patients similar to the present study.
Keywords: Persistent Neuropathic Pain; Intramedullary Hemorrhage; Spinal Dural Arteriovenous Fistula; Dorsal Root Entry Zone
Introduction
Spinal dural arteriovenous fistulas (SDAVFs) are the most common type of spinal vascular malformations. Typically, the patients with SDAVFs present with chronic progressive myelopathy, resulted from venous congestion or hypertension [1,2]. Hemorrhage from SDAVFs is usually rare and may occur as subarachnoid hemorrhage (SAH) from the fistulas in cervical or cranio-cervical region [3-5]. Intramedullary hemorrhage or hematomyelia caused by SDAVFs is extremely rare. The incidence of hemorrhage in thoracolumbar DAVFs was less than 1% [6]. We reported intramedullary hemorrhage in the young woman with SDAVF. The major problem in this patient was persistent at-level neuropathic pain even after surgical obliteration of the fistula and dorsal root entry zone (DREZ) lesioning. In addition, we also reviewed the literature of intramedullary hemorrhage related to SDAVFs.
Case Presentation
A 34-year-old woman suffered from sudden severe electric-like pain and paresthesia at the left anterior and posterior chest wall below nipple line during working in her office. There was no muscle weakness or bowel/ bladder dysfunction involving. She was sent to the emergency department of the nearby private hospital. The initial diagnosis was neuralgia of chest wall which could be partially relieved by pregabalin 75mg twice a day and tramadol 50mg every 6 hours as needed for pain. She was admitted for observation and discharged home 3 days later. Due to progressive pain, she went back to the private hospital 2 weeks later and obtained magnetic resonance imaging (MRI), revealing intramedullary hemorrhage, which showed heterogeneous signal intensity on T2 weighted images, extending from the level of lower T5 to upper T7 of the left side of the spinal cord. There was abnormal intradural flow voids along left posterolateral cord surface from the level of T6 to T11. Magnetic resonance angiography (MRA) demonstrated apparently enlarged and tortuous ascending and descending draining perimedullary veins (Figure 1). She was transferred to Prasat Neurological Institute for further investigation and treatment. On physical examination, the only abnormal finding of neurological examination was decreasing of pin-prick sensation over the left T6 dermatome. Due to young age and manifesting as hematomyelia, initial diagnosis of this patient was ruptured spinal cord arteriovenous malformation (AVM), cavernoma, or rarely perimedullary AVF. In fact, spinal angiography revealed SDAVF, fed mainly by radiculomeningeal branches from the left T6 intercostal artery with drainage into ascending and descending prominent and tortuous perimedullary draining veins (Figure 2A, 2B). Additionally, the left T5 intercostal artery also gave radiculomeningeal branch to the fistula (Figure 2C). 3D rotational angiography and axial maximum intensity projection (MIP) reformatted image of angiographic CT from the left T6 intercostal artery injection confirmed a small venous varix, suspected from T5 and T6 intercostal artery injections (Figure 2A, 2C, 2D, and 2E).The artery of Adamkiewicz arose mainly from the left T8 intercostal artery (Figure 2F) and also contributed from the left T6 intercostal artery, clearly seen by superselective angiography with the microcatheter (Figure 2G). The left T6 intercostal artery not only gave rise the branch to the fistula, but also anterior spinal artery. Therefore, endovascular treatment with liquid embolic material was contraindication for this patient. Decision making of surgery for obliteration the fistula was recommended to the patient. However, she hesitated and refused the surgery in this admission. Later, she went to King Chulalongkorn Memorial Hospital for the second opinion, and received the same plan of treatment. Six months after initial symptoms, she underwent thoracic laminectomy from T5 toT7 with microsurgical obliteration of the fistula. The complete occlusion of the fistula was confirmed by intraoperative angiography using indocyanine green. DREZ lesioning of the left T6 root was also performed in the same session.
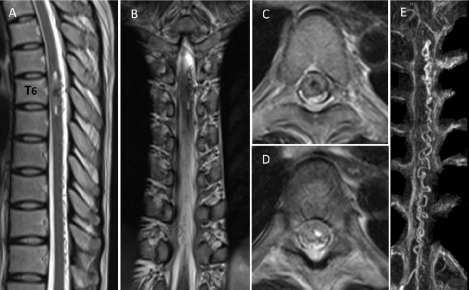
Figure 1: The left T6 intercostal artery injections in arterial (A) and venous (B) phase showed a spinal dural arteriovenous fistula, supplied by radiculomeningeal
branches from the left T6 intercostal artery with drainage into ascending and descending perimedullary veins. Small venous varix (black arrowhead) was suspected.
The left T5 intercostal artery injection (C) demonstrated that radiculomeningeal branches from this level also gave rise to the fistula with suspected small venous
varix (black arrowhead). 3D rotational angiography (D) and axial maximum intensity projection (MIP) reformatted image of angiographic CT (E) at the level of the
left T6 intercostal artery confirmed the small venous varix (white and black arrowheads). The left T8 intercostal artery injection (F) revealed a radiculomedullary
branch that contributes to the anterior spinal artery (the artery of Adamkiewicz). Superselective injection of the left T6 intercostal artery (G) by the microcatheter
demonstrated another additional contribution of the artery of adamkiewicz.
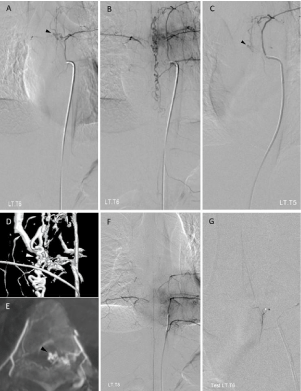
Figure 2: The left T6 intercostal artery injections in arterial (A) and venous (B) phase showed a spinal dural arteriovenous fistula, supplied by radiculomeningeal
branches from the left T6 intercostal artery with drainage into ascending and descending perimedullary veins. Small venous varix (black arrowhead) was suspected.
The left T5 intercostal artery injection (C) demonstrated that radiculomeningeal branches from this level also gave rise to the fistula with suspected small venous
varix (black arrowhead). 3D rotational angiography (D) and axial maximum intensity projection (MIP) reformatted image of angiographic CT (E) at the level of the
left T6 intercostal artery confirmed the small venous varix (white and black arrowheads). The left T8 intercostal artery injection (F) revealed a radiculomedullary
branch that contributes to the anterior spinal artery (the artery of Adamkiewicz). Superselective injection of the left T6 intercostal artery (G) by the microcatheter
demonstrated another additional contribution of the artery of adamkiewicz.
The previous chest pain preoperatively was totally relieved for a few days after surgery. The at-level neuropathic pain gradually returned with stabbing, cramping, and itching sensation. On the fifth postoperative day, her pain score was 10/10. The pain affected her daily life, and was precipitated by deep breathing and movement. Gabapentin, etoricoxib, and tramadol were given to ease her pain. But the pain could not be well controlled. The pain was severe enough that she could not go back to work. Follow- up spinal angiography 2 months after operation revealed no residual AVF (Figure 3A).
Two months after operation, the patient was sent to the pain clinic of King Chulalongkorn Memorial Hospital. The diagnosis of mixed pain from chronic postsurgical pain at the healed surgical site T5-T7 and left T6 neuropathic pain was done (Figure 4). The painaggravating factors were premenstrual period, stress, mechanical pressure, and fear of untreatable pain. The pain- relieving factors were warm bath and gentle rub. Thermal quantitative sensory testing (QST) tests were performed using the Medoc TSAII Neurosensory Analyzer device (Medoc Ltd. Advanced Medical Systems, Ramat Yishai, Israel) by comparison between the pain area at the left anterolateral chest wall and the contralateral control area. The QST study showed significant decrease in cold detection threshold (mean, 20.2ºC VS 28.7ºC) and cold pain threshold (mean, 1.3ºC VS 24.7ºC), and moderate increase in warm detection threshold (mean, 37.0ºC VS 35.1ºC) and heat pain threshold (mean, 46.6ºC VS 37.8ºC). The results of left and right side QST at the back of T6 dermatome were cold detection threshold of mean 27.5ºC VS 27.0ºC, cold pain threshold of mean 1.2ºC VS 21.9ºC, warm detection threshold of mean 34.6ºC VS 34.1ºC, and heat pain threshold of mean 42.6ºC VS 37.4ºC. The data indicated abnormal non-nociceptive and nociceptive thermal sensitivity. Pregabalin starting at the dosage of 75mg per day was increasing to 600mg per day in one and a half month. After having pregabalin, she observed that the pain became to be itching before having much pain relief. Amitriptyline 10mg every night at bedtime was also given to her. She could not tolerate the side effects of amitriptyline. Then, nortriptyline was prescribed instead starting with 10mg. Her pain was in controlled by pregabalin 525mg per day, nortriptyline 25mg, 5% lidocaine patch, and tramadol 50mg as rescue medication once a month. Two months after adjusting drugs by the pain specialist, she could go back to work with a pain free period for only 1 month. Subsequently, she has regained the pain from time to time, especially when she was in stress. Her pain score at the chest wall was decreased to 3-4/10. The dosage of pregabalin has gradually been decreased. Follow-up MRI one year after operation showed disappearance of blood components in spinal cord without spinal cord atrophy (Figure 3B-3D). At 2 years after operation, the pain was controlled in acceptable level with pregabalin 150mg twice a day, nortriptyline 25mg at bedtime, and 5% lidocaine patch, applied over the painful chest wall.
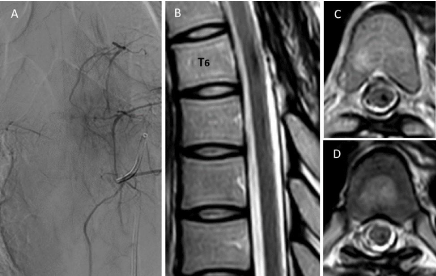
Figure 3: Spinal angiography obtained two months after the operation. Left T6 intercostal artery injection (A) confirmed complete obliteration of the fistula and a
patent of the artery of Adamkiewicz. Magnetic resonance imaging obtained one year after the operation. Sagittal (B) and axial T2-weighted images at level of midvertebral
body of T6 (C) and T6-T7 (D) revealed complete resolution of blood and disappearance of abnormal flow voids without spinal cord atrophy.
Discussion
SDAVFs, also referred to as type I spinal AVMs, and intradural dorsal AVFs, are the shunts supplied by radiculomeningeal arteries draining into the radicular veins arterializing the coronal venous plexus around the spinal cord, usually under low pressure, resulting in venous congestion [1,2,7]. The fistulas usually locate intradural dorsal aspect close to the root sleeves and frequently occur in the thoracolumbar region [2]. These fistulas, low-flow, are the most common type of spinal vascular malformations and have been subdivided into common type A, i.e. single feeding artery, and rare type B, i.e. multiple feeding arteries [1]. The patients, typically elderly men, commonly manifest with gradual progression of myelopathy induced by chronic venous hypertension [8]. Clinical symptoms include back pain, lower legs pain, paraparesis, paresthesia, impotence, and/or bowel/bladder dysfunctions. The characteristic findings on T2-weighted sequences in MRI are combination of spinal cord edema and perimedullary flow voids [2].
We reviewed the literature of SDAVFs with intramedullary hemorrhage (Table 1) [6,9-12]. There were only 6 cases, including our one case. They were 4 males and 2 females with a median age of 50 years (IQR (p25-p75) 35.5-68 years). All fistulas were located at thoracic region. Three fistulas were located on the right side, and three on the left side. Most fistulas have one feeding artery (type A), except our case has 2 feeding arteries (type B). All but one of the SDAVFs have venous varices of draining veins, being source of intramedullary hemorrhage. Most patients have symptoms and signs of progressive myelopathy, bowel or bladder dysfunction, and/or sensory disturbance. Interestingly, the symptoms of our patient were pain and paresthesia of left T6 dermatome without neurological deficits. Another 3 patients have presenting symptoms of pain, including epigastric pain, chest pain, back pain, and abdominal pain. Four cases were treated by surgery, and another two cases by embolization. After treatment, most patients have limited postoperative functional recovery. Regarding postoperative pain, only our case has persistent pain at an area of the left T6 dermatome.
Authors
Sex/Age
Symptoms and signs
Fistula Side
Feeding artery
Cause of hemorrhage
Treatment
Spinal cord atrophy
Outcome
Mascalchi, 1998 [16]
M/74
Paraplegia, sensory loss below T11, sphincter incontinence
R
T8
Venous varix
Embolization
Yes
No changes of neurological deficit.
Minami, 2009 [17]
M/51
Epigastric pain, paraplegia, and sensory loss below nipples
R
T7
Venous varix
Surgery
N/A
No functionally significant improvement.
Narisawa, 2014 [18]
M/49
Intermittent stabbing chest pain, back pain, left leg weakness, sensory loss below T12
L
T6
Venous varix
Surgery
N/A
Chest pain subsided immediately after the operation. He still required a cane for ambulation, and needed self-catheterization for voiding urine at two years follow-up.
Schmidt, 2014 [22]
M/36
steroid-induced worsening, severe spastic paraplegia, sensory loss below T12
L
T3
Severe venous hypertension
Surgery
N/A
Continued improvement, including the ability to take a few step unaided at 2 months follow-up.
Hamdan, 2015 [8]
F/66
Abdominal pain, paraplegia, sensory loss below the costal margins, urinary retention
R
T7
Venous varix
Embolization
N/A
Remained unchanged after the procedure and 1 month before discharge for further rehabilitation.
Present study, 2019
F/34
Left T6 radiculopathy
L
T5, T6
Venous varix
Surgery
No
Persistent neuropathic pain at one year follow-up.
F: female; M: male; L: left; R: right; T: thoracic; N/A: not applicable
Table 1: Literature review of spinal dural arteriovenous fistula with intramedullary hemorrhage.
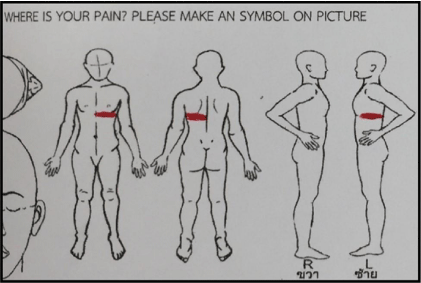
Figure 4: The perceived pain area, drawn by the patient, was at anterior and posterior of left chest wall below the nipple, corresponding with the left T6 dermatome.
Since 1998, first patient with intramedullary hemorrhage caused by SDVAF was reported by Mascalchi, et al. [9]. Unequivocally, the cause of hemorrhage resulted from a round-shape aneurysmal like ectasia. A decade later, Minami, et al. [10] demonstrated the patient presenting with hematomyelia and SAH secondary to ruptured intraparenchymal venous varix of SDAVF, confirmed by intraoperative findings. In addition, they also speculated that thrombosis in draining veins, including the varix, may possibly increase the risk of rupture. Subsequently, Narisawa, et al. [11] clearly revealed rupture of a varix, embedded into the spinal cord parenchyma caused hematomyelia by evidences from images, intraoperative and pathological findings. Previous T2-weighted image also revealed hyperintensity area in the spinal cord around a wedged portion of the venous varix, probably representing an increase in transluminal pressure and predicted hemorrhagic event. Another case report of intramedullary hemorrhage due to SDAVF by Hamdan, et al. [6], they also speculated that an increased venous flow into a varix may be considered an important risk factor of hemorrhage. Without the presence of a venous varix, Schmidt, et al. [12] suspected that intramedullary hemorrhage, occurred in the area of cord edema, may result from severe venous hypertension rather than hemorrhagic infarction of the spinal cord. They found that the patient significantly improved following surgical obliteration of the fistula even with longstanding severe neurological deficits. Recently, we also found that the possible cause of intramedullary hemorrhage from SDAVF resulted from the venous varix, confirmed by 3D rotational angiography.
Hematomyelia commonly tends to extend multi-segments rostrally and caudally from the area of initial hemorrhage, involving mainly in the gray matter and the surrounding white matter. The hematoma may be localized within the spinal cord substance, subarachnoid, and/or subdural spaces [13-15]. As seen on MRI in our case, intramedullary spinal cord hemorrhage dissected longitudinally above and below the area of initial hemorrhage.
SDAVFs can be treated by surgery, endovascular treatment, or both depend on institutions preference [1,2,8,13]. The goal of treatment of SDAVF is to obliterate the fistula, including the proximal portion of the draining vein, without excision of the dilated venous plexus [7]. In our patient, she was decided to perform surgery because the artery of Adamkiewicz or radiculomedullary artery originating from the same level of the fistula is a contraindication for endovascular treatment [16].
According to the study of microsurgical DREZotomy for neuropathic pain due to spinal cord injuries in long term follow-up by Sindou, et al. [17], they found that the patients with segmental pain obtained good results much more than in patients with predominant below-level pain. In addition, better outcomes were obtained in the patients with paroxysmal attacks of pain like electric shocks than in those with continuous spontaneous burning pain. Due to intractable neuropathic pain in our patient, DREZ lesioning was considered to be performed in the same session. However, following these procedures, the pain in T6 dermatome has still persisted.
In the clinical features of non-traumatic intramedullary hemorrhage, sudden onset of intense pain is usually associated with severe neurological deficit or progressive neurological deterioration [15]. However, atypical manifestation of SDAVF in our patient may result from small focal area of hemorrhage abutting dorsal T6 root exit zone without venous congestion.
Chronic neuropathic pain is a common and significant problem occurring following spinal cord injury, leading to significantly interfere with daily functioning [18,19]. Central neuropathic pain can result from any lesions along the spinothalamocortical pathways of brain and spinal cord, more frequent, by trauma or disease. This type of neuropathic pain is characterized by loss of sensations and abnormal pain perception in the painful skin areas. Patients with this pain may complain of different symptoms. A burning, icelike sensation is frequently reported. Pain onset may be noticed immediately or delayed [20]. In thoracic level, the character of pain may be a feeling of tightness or burning in dermatomal bands at the chest or abdomen [21].
Siddall, et al. [22] longitudinally studied the prevalence and characteristics of pain following spinal cord injury, and found that the pain was present in 81% and identified into four different main types, including musculoskeletal, visceral, at-level neuropathic, and below-level neuropathic pain. At-level neuropathic pain was present in 41%, and approximately half of the patients with this type of pain presented for the first time within the first 3 months after injury. These patients are likely to continue to experience ongoing pain and the pain is likely to be both severe and persistent. Moreover, chronic neuropathic pain following injury is associated with psychological distress and response poorly to currently available treatments. Segmental or at-level neuropathic pain is the pain resulted from damage to the substance of the spinal cord and probably arise from generators or neoformed local networks located in the DREZ and/ or dorsal horn region [17,21]. Conventional pharmacological and surgical pain therapies for this pain is usually ineffective [23].
Central neuropathic pain in patients with SDAVF may resulted from irreversible spinal cord changes caused by chronic venous congestion. In the course of long- term follow-up, Sasamori, et al. [8] surveyed chronic pain due to SDAVF and found that chronic leg pain, diffuse and bilateral, was reported by 81.3% of all patients. These patients experienced moderate to severe pain either before treatment or new onset after treatment. Assessment by the neuropathic pain symptom inventory (NPSI), the subscores were significantly higher for spontaneous pain and paresthesia/dysesthesia than for paroxysmal pain. They also found that Spinal cord atrophy on MRI follow-up was a specific finding in patients having chronic pain. Shinoyama, et al [24] studied outcomes of thoracolumbar DAVF with emphasis on neuropathic pain and speculated that worsening or aggravating of neuropathic pain may correlated with spinal cord atrophy and a residual intramedullary hyperintense lesion in T2 weighted MRI. However, there was no spinal cord atrophy on MRI follow-up in our patient.
The diagnosis of pain type from clinical findings and evaluation of the effect of treatment are imperative for the management of neuropathic pain following spinal cord injury. Pharmacological treatment of pain in this condition is long-running process and this pain responds poorly to monotherapy. Currently, the first-choice drugs having the best documented effect on neuropathic pain include a tricyclic antidepressant (amitriptyline), and antiepileptic drug (pregabalin and gabapentin). The applied doses for amitriptyline varied between 10 and 150 mg, pregabalin 150 and 600 mg, and gabapentin 300 and 3600 mg [25]. In treatment of neuropathic pain related to spinal cord injury, gabapentin can reduce the intensity as well as the frequency of pain, and improve the quality of life. Gabapentin provided statistically significant alleviation for all neuropathic pain descriptors, except itchy, dull, sensitive, and cold types. Adverse events of this drug, including somnolence, dizziness, weakness, edema, vertigo, and headache, have been reported as minor and well tolerated [18]. In patients with central neuropathic pain associated with spinal cord injury, pregabalin 150 to 600 mg per day was significantly more effective than placebo in relieving moderate to severe pain, improving sleep, anxiety, and overall patient status. The most common adverse events of pregabalin were somnolence, dizziness, and peripheral edema [26]. Tramadol might be used as the second-line treatment and can decrease pain intensity. However, it is associated with the adverse events, commonly in tiredness, dry mouth, and dizziness. To minimize the risk of adverse events, titration should be slow and individual [27]. Topical agents, such as 0.025% capsaicin ointments or lidocaine, may be useful [25].
Conclusion
The authors report a patient with chronic at-level neuropathic pain secondary to intramedullary hemorrhage from SDAVF. Surgical obliteration of the fistula and DREZ lesioning were performed in the same session. Follow-up images confirmed complete occlusion of the fistula and disappearance of blood components. Although at-level severe neuropathic pain has been a favorable indication for DREZ lesioning and performing this procedure following surgical obliteration of the fistula was an option, our patient still has suffered from the pain. Despite having received multi-drug therapy, the acceptable neuropathic pain has remained at 2 years after operation.
References
- Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002; 96: 145-156.
- Krings T, Lasjaunias PL, Geibprasert S, Hans FJ, Thron AK, terBrugge KG, Reinges MH. Classification of Spinal Vascular Malformations. The Neuroradiology Journal. 2009; 22: 97-106.
- Kinouchi H, Mizoi K, Takahashi A, Nagamine Y, Koshu K, Yoshimoto T. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg. 1998; 89: 755-761.
- Aviv RI, Shad A, Tomlinson G, Niemann D, Teddy PJ, Molyneux AJ, Byrne JV. Cervical dural arteriovenous fistulae manifesting as subarachnoid hemorrhage: Report of two cases and literature review. AJNR Am J Neuroradiol. 2004; 25: 854-858.
- Kai Y, Hamada J, Morioka M, Yano S, Mizuno T, Kuratsu J. Arteriovenous fistulas at the cervicomedullary junction presenting with subarachnoid hemorrhage: Six case reports with special reference to the angiographic pattern of venous drainage. AJNR Am J Neuroradiol. 2005; 26: 1949-1954.
- Hamdan A, Padmanabhan R. Intramedullary hemorrhage from a thoracolumbar dural arteriovenous fistula. Spine J. 2015; 15: e 9-16.
- Anson JA, Spetzler RF. Interventional neuroradiology for spinal pathology. Clin Neurosurg. 1992; 39: 388-417.
- Sasamori T, Hida K, Osanai T, Yano S, Seki T, Houkin K. A Survey of Chronic Pain Due to Spinal Dural Arteriovenous Fistulae. Neurosurgery. 2015; 77: 113-118.
- Mascalchi M, Mangiafico S, Marin E. Hematomyelia complicating a spinal dural arteriovenous fistula. Report of a case. J Neuroradiol. 1998; 25: 140- 143.
- Minami M, Hanakita J, Takahashi T, Kitahama Y, Onoue S, Kino T, Ito K, Ezaki Y. Spinal dural arteriovenous fistula with hematomyelia caused by intraparenchymal varix of draining vein. Spine J. 2009; 9: e15-19.
- Narisawa A, Endo T, Sato K, Watanabe M, Takahashi A, Tominaga T. Spinal dural arteriovenous shunt presenting with intramedullary hemorrhage: Case report. J Neurosurg Spine. 2014; 20: 322-326.
- Schmidt KA, Huang JF,Black DF, Kaufmann TJ, Lanzino G, Kumar N. Spinal dural arteriovenous fistula with intramedullary cord hemorrhage: Diagnostic challenges. Neurol Clin Pract. 2014; 4: 486-489.
- Leech RW, Pitha JV, Brumback RA. Spontaneous haematomyelia: A necropsy study. J Neurol Neurosurg Psychiatry. 1991; 54: 172-174.
- Karavelis A, Foroglou G, Petsanas A, Zarampoukas T. Spinal cord dysfunction caused by non-traumatic hematomyelia. Spinal Cord. 1996; 34: 268-271.
- Leep Hunderfund AN, Wijdicks EF. Intramedullary spinal cord hemorrhage (hematomyelia). Rev Neurol Dis. 2009; 6: E54-61.
- Rodesch G, Lasjaunias P. Spinal cord arteriovenous shunts: From imaging to management. Eur J Radiol. 2003; 46: 221-232.
- Sindou M, Mertens P, Wael M. Microsurgical DREZotomy for pain due to spinal cord and/or cauda equina injuries: Long-term results in a series of 44 patients. Pain. 2001; 92: 159-171.
- Turner JA, Cardenas DD. Chronic pain problems in individuals with spinal cord injuries. Semin Clin Neuropsychiatry. 1999; 4: 186-194.
- Davidoff G, Roth E, Guarracini M, Sliwa J, Yarkony G. Function-limiting dysesthetic pain syndrome among traumatic spinal cord injury patients: A cross-sectional study. Pain. 1987; 29: 39-48.
- Devulder J, Crombez E, Mortier E. Central pain: An overview. Acta Neurol Belg. 2002; 102: 97-103.
- Bryce TN, Ragnarsson KT. Pain after spinal cord injury. Phys Med Rehabil Clin N Am. 2000; 11: 157-168.
- Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003; 103: 249-257.
- Eide PK. Pathophysiological mechanisms of central neuropathic pain after spinal cord injury. Spinal Cord. 1998; 36: 601-612.
- Shinoyama M, Endo T, Takahash T, Shimizu H, Takahashi A, Suzuki M, Tominaga T. Long-term outcome of cervical and thoracolumbar dural arteriovenous fistulas with emphasis on sensory disturbance and neuropathic pain. World Neurosurg. 2010; 73: 401-408.
- Hagen EM, Rekand T. Management of Neuropathic Pain Associated with Spinal Cord Injury. Pain Ther. 2015; 4: 51-65.
- Levendoglu F, Ogün CO, Ozerbil O, Ogün TC, Ugurlu H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine (Phila Pa 1976). 2004; 29: 743-751.
- Norrbrink C, Lundeberg T. Tramadol in neuropathic pain after spinal cord injury: A randomized, double-blind, placebo-controlled trial. Clin J Pain. 2009; 25: 177-184.
Citation: Iampreechakul P, Lertbutsayanukul P, Siriwimonmas S, Jittapiromsak P, Tantivatana J and Niruthisard S. Persistent Central Neuropathic Pain Caused by Intramedullary Hemorrhage from Spinal Dural Arteriovenous Fistula: A Case Report and Literature Review. Austin J Anesthesia and Analgesia. 2019; 7(1): 1076.