Case Presentation
Ann Surg Perioper Care. 2016; 1(2): 1007.
Massive Facial Neurofibromas: Review of the Literature, New Classification System and Case Presentations
Bogdasarian RN¹, Fientisch AM¹, Langer PD² and Datiashvili RO¹*
¹Department of Surgery, Division of Plastic Surgery, Rutgers State University of New Jersey/New Jersey Medical School, USA
²Department of Ophthalmology and Visual Science, Rutgers State University of New Jersey/New Jersey Medical School, USA
*Corresponding author: Datiashvili RO, Department of Surgery, Division of Plastic Surgery, Rutgers State University of New Jersey/New Jersey Medical School, ACC Building E-1620, NewYark, NJ 07103, USA
Received: October 05, 2016; Accepted: October 21, 2016; Published: October 24, 2016
Abstract
Massive facial neurofibromas are rare, occurring in 1 in 300,000 individuals.
In the presented article, we review the literature and our recent experience with MFNs. We provide an overview of management strategies and develop a classification system and treatment algorithm based on modern treatment options.
In this comprehensive review, Ovid Medline and the Cochrane library were mined for the relevant literature. Articles were extracted from the bibliographies of selected papers. The literature search returned 52 articles including literature reviews, case series and case studies.
We developed a three tiered classification system, based on the anatomical structures affected by the MFN. Our treatment algorithm provides concise guidelines for preoperative planning.
Keywords: Neurofibromatosis; Facial Neurofibroma; Surgery; Treatment; Classification; Algorithm
Abbreviations
MFN: Massive Facial Neurofibroma; NF1: NeuroFibromatosis Type One
Introduction
Massive facial neurofibromas (MFNs) pose formidable challenges to the surgeon: aesthetic, functional, reconstructive, and risk of severe bleeding. We define MFNs as neurofibromas involving greater than 25% of the face.
MFNs are associated with Neurofibromatosis Type I (NF1) or Von Recklinghausen Syndrome, an autosomal dominant disorder of variable penetrance resulting in benign, yet often disfiguring hamARtomas of peripheral nerve sheaths. NF1 affects 1/3000 individuals. Of these individuals, 1% is affected by MFNs, or 1/300,000 of the general population.
MFNs are variable, most often located in the orbitotemporal region, but with a wide range of effects on skin quality and special structures such as the orbit, eye, lids, lips, oral commissure, facial muscles, nerves, skeleton, and so on. All MFNs are highly vascular, amorphous tumors. They are prone to recurrence, have potential for malignant transformation (3.5% in the adult), and are located in cosmetically sensitive areas [1-6]. A consensus, standardized management protocol does not exist. The algorithms that do exist are restricted to specific anatomical regions and lack guidelines for a comprehensive medical and surgical approach. Thus, outcomes may benefit from a comprehensive classification system and treatment algorithm to guide preoperative planning.
MFNs in children may demonstrate rapid growth and malignant transformation (up to 20% in one study), and thus should be biopsied and closely observed [7]. Resection of MFNs in children is typically deferred until the age of 18 or until the MFN stabilizes, unless evidence of malignancy presents. This review focuses on the treatment of adults.
The purpose of this article is to share a review of the literature and our experience with two cases of MFNs. We developed and present a new classification system and treatment algorithm for preoperative planning.
Case I
A 29 year old male with history of NF1 and prior tumor resection of the scalp presented with left sided MFN involving the temporopalpebral region and cheek (Figure 1, Left). Paresis of the frontal branch of the facial nerve and left eye blindness were present. There was a relatively good skin condition overlying the tumor (type Ia, IIb, IIIa; see Table 1). The patient underwent a two-stage reconstruction consisting of primary debulking of the tumor with local skin flap arrangement with subsequent secondary canthopexy and browpexy. The highly vascular nature of the patient’s tumor resulted in moderate blood loss, especially during the primary procedure. Bleeding was controlled with pressure, electrocautery, and clip ligation. Preoperative tumor embolization, banked autologous blood and cross-matched blood ensured the patient’s safety. There were no postoperative complications (Figure 1, Right) [8]. The patient is very satisfied with his outcome.
Class
Description
Treatment
Ia
Bone not significantly Involved
Soft tissue surgery
Ib
Bone significantly involved
Neurosurgery, ENT, Oculoplastics collaboration
IIa
Lids, nose, lips, ears spared
Single surgery
IIb
Lids, nose, lips, ears involved
Staged surgeries
IIIa
Satisfactory tumor skin quality
Local flap reconstruction
IIIb
Poor tumor skin quality, facial structure intact
Skin grafting, local flaps, free flap, dermabrasion, tissue expansion
IIIc
Poor tumor skin quality, facial structure obliterated
Free flap, face transplant
Table 1: MFN Classification System and treatment correlates. Each case receives three classification designations from each section (I, II, III) of the classification system (e.g. a patient with uninvolved bone, spared special structures and satisfactory overlying skin is designated as: Ia, IIa, IIIa).
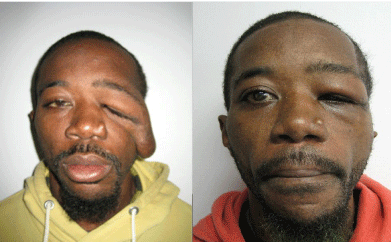
Figure 1: Left – preoperative view. Right – postoperative view after staged
resections, canthopexy, browbexy, and adjacent tissue transfers. Permission
obtained from E-Plasty for use of images.
neurofibroma. The patient exhibited a giant 30x25cm neurofibroma involving the left lower eyelid, cheek, nose, and upper and lower lips (Figure 2). The patient had asymptomatic absence of the left sphenoid greater wing. Features included sufficient skin of satisfactory quality overlying the tumor (type Ia, IIb, IIIa; see Table 1). The patient had been seeking treatment for many years, but unsuccessfully.
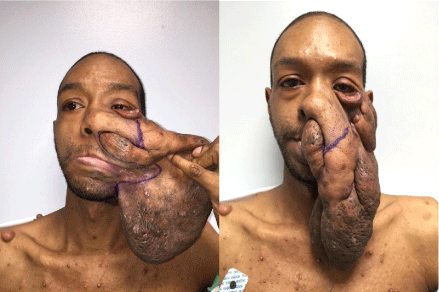
Figure 2: Preoperative Views: Frontal view with tumor retracted (left) and
tumor in repose (right).
Preoperative CTA (Figure 3) and MRI (Figure 4) showed large vessels feeding the tumor. Selective arterial embolization was performed 24 hours prior to surgery. Autologous and cross-matched blood was on hold and a cell saver autologous blood recovery system (Haemonetics, Braintree, MA) was prepared. Resection of the tumor was performed using the PEAK plasma blade (Medtronic, Minneapolis, MN) and Ligasure small jaw open instrument (Medtronic, Minneapolis, MN) for effective hemostasis. Only massive debulking and suture suspension of the left nasal ala were performed at this first stage.
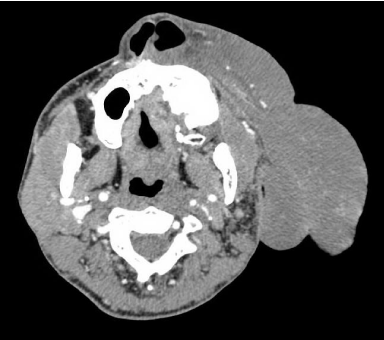
Figure 3: Pre-Operative CTA. Extensive vascular supply arising from multiple
branches of the external carotid artery.
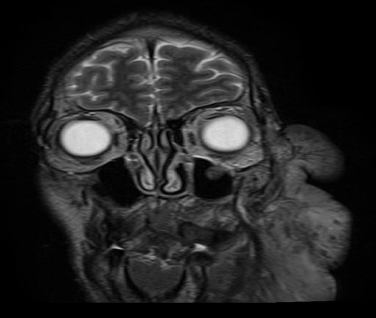
Figure 4: Preoperative MRI. Large plexiform mass arising from the left
periorbital, nasal, premaxillary, and perimandibular soft tissues. Bony atrophy
is noted.
Five months later, the second surgery was performed. This included excision of the left lower eyelid and orbital tumor, conjuctivoplasty and canthopexy (Figure 5).
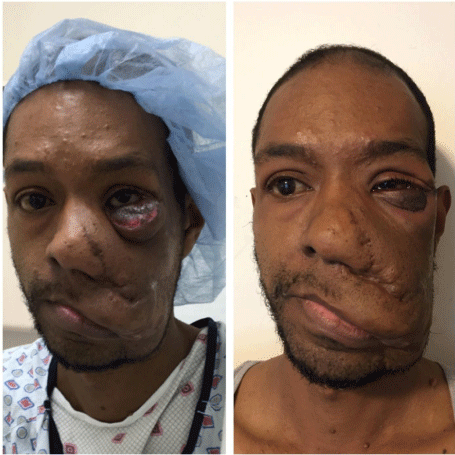
Figure 5: Left – Prior to second stage, 5 months after initial tumor debulking.
Right – Immediately post second stage excision of the left lower eyelid and
orbital tumor, conjuctivoplasty and canthopexy.
Material and Methods
In this comprehensive review of the literature, Ovid Medline was used with the MESH terms neurofibroma OR neurofibromatosis AND face OR craniofacial abnormalities OR orbital neoplasms OR orbits OR head OR head and neck neoplasms. The search was restricted to literature from 1995 to the present, English language, human subjects, systematic reviews, reviews, randomized controlled trials, clinical trials, meta-analyses, practice guidelines and case reports. The search resulted in 458 articles of which 43 were selected by the authors for relevance to the topic. Additional articles were extracted from bibliographies of prior review articles using the PubMed citation matcher. The Cochrane Library was explored using several search terms similar to facial neurofibroma.
Relevant articles were chosen based on the description and treatment of MFNs involving the hard and soft tissues of the head and neck. We excluded articles on intranasal, sinus, laryngeal or brain invasion of tumor which fall outside of the scope of the common plastic surgery practice.
An MFN classification system was formulated based on the treatment strategies found to be most successful in the literature with input from our experience.
Results and Discussion
The literature search returned 52 articles including nine literature reviews, 30 case series, 12 case studies, and one multicenter study. Nine articles offered classifications or specific treatment recommendations. However, none of these offered a comprehensive treatment algorithm. The Cochrane Library review returned no articles.
Based on the literature review and our clinical experience, we developed a comprehensive MFN Classification System (Table1) and Treatment Algorithm (Figure 6) is based on tumor characteristics found to be determining factors for treatment approach. These systems may be used to guide preoperative planning.
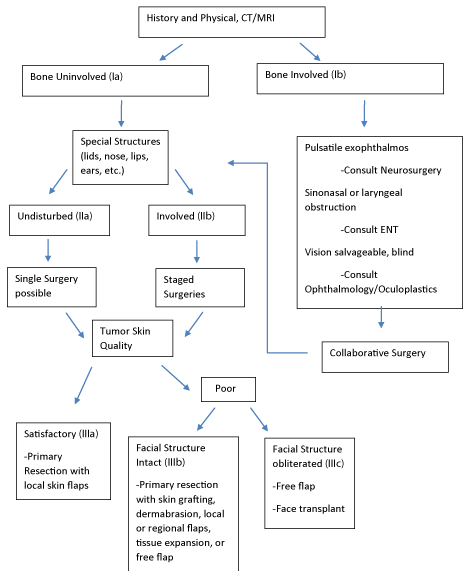
Figure 6: MFN Treatment Algorithm. Derived from MFN Classification System. A guide for preoperative planning.
Preoperative Planning
Reconstructive, functional, and aesthetic outcomes, as well as control of anticipated complications including massive bleeding, seroma, and scarring depend on preoperative planning. Management may be guided and enhanced by adhering to the algorithm found in figure 6..
Management of Skeletal Defects: Class I
Skeletal reconstruction is the first consideration in the treatment algorithm. MFNs are associated with skeletal defects of the head and neck. Major bone involvement may result in pulsatile exophthalmos, enophthalmos, sinusitis, external ear canal disease or herniation of brain matter. Skeletal reconstruction is the primary consideration of the algorithm in order to emphasize the anatomical and functional importance of skeletal structure to establish a stable foundation for overlying soft tissues.
A patient with asymptomatic bone anatomy is classified Ia. The patient with symptomatic bone defects requiring bone reconstruction is classified Ib. Early collaboration with neurosurgery, otolaryngology, oral maxillofacial surgery, oculoplastics and/or ophthalmology is encouraged for treatment planning and execution for patients falling into class Ib [9-14]. Class Ia may be managed by the plastic surgeon alone.
Several treatment strategies exist, differing between children and adults. Titanium mesh is usually implanted without bone grafts in adults due to unacceptably high bone resorption rates [15]. Bone grafts from the cranium, iliac crest, or rib may be placed with titanium mesh reinforcement in the young. Ophthalmology assessment is mandatory to assess vision in all cases involving the orbit [10,15-20].
Surgical Staging: Class II
The second tier of the classification system focuses on the special structures of the face. The patient without symptomatic involvement of the eyelids, lips, nose, or ears is classified IIa. For these patients, a single surgery to debulk the tumor may suffice.
Frequently, MFN patients present with symptomatic involvement of the special structures of the face, and require staged procedures to retain or restore function, or to improve aesthetics. These patients are classified as IIb. Functional outcomes depend heavily on preoperative function. Blindness, facial muscle weakness or paralysis, nasal valve collapse, and oral incompetence are common due to tumor mass effect, invasion of nerves or facial spaces, and attenuation of muscles due to gravitational force. A preoperative MRI will demonstrate the extent of tumor invasion and proximity to subcutaneous structures and can be valuable for resection planning [10,20,22]. The literature supports a debulking operation as a primary procedure for development of a stable soft tissue platform [13,21].
The primary operation is followed by secondary procedures focused on the special structures in order to obtain maximal form and function. The primary surgery not only removes tumor, but also establishes a favorable hard and soft tissue foundation and sets the stage for secondary procedures.
Aesthetic and social concerns are managed through a discussion with the patient to ensure that he or she has rational expectations. Patients benefit from viewing pictures from prior cases and having all questions answered [3]. An understanding of functions that may be improved (oral competence, nasal airflow) versus those that cannot be recovered (vision, sensorineural hearing) is essential. The plan for staged procedures must be discussed early with the patient.
Surgical Technique for Primary Debulking
We recommend the patient is admitted for selective arterial embolization of the tumor within 48 hours of surgery. Autologous and type and cross-matched blood and the cell saver autologous blood recovery system (Haemonetics, Braintree, MA) should be on hand in anticipation of large blood loss.
The day of surgery, the patient is marked by the surgical team while awake so that expectations can be reviewed with the patient. The creativity of the surgeon is employed during markings, taking into consideration the facial subunits affected and the skin quality overlying the tumor and around it. The contralateral normal facial subunits are used as a template. Any possible scenarios of the procedure such as skin grafting, dermabrasion, tissue expansion or flaps are discussed and consented for.
Once in the operating room under general anesthesia, the patient’s entire face is prepped and draped so that the contralateral anatomy can be appreciated during resection and reconstruction.
Lidocaine with epinephrine is injected into incision sites for hemostasis. The PEAK plasma blade (Medtronic, Minneapolis, MN) is used to make incisions and cauterize. The Ligasure small jaw open instrument (Medtronic, Minneapolis, MN) is used for the majority of the resection, resulting in excellent hemostasis.
Normal tissue planes usually do not exist and one should expect to leave behind a layer of tumor within the skin flaps overlying the attenuated facial musculature. This improves vascularity to the skin flaps, and protects the facial nerve branches. For auricular MFNs, nerve monitoring is recommended [15]. If radical tumor ablation is planned, a free flap reconstruction is nearly mandatory.
The tumor is debulked without compromising the valuable skin and subcutaneous tissues around the eyes, nose, mouth and ear, even if these structures are infiltrated by tumor [21].
Once satisfied with the resection, skin flaps are revised as necessary. Suspension procedures are performed to achieve the stable soft tissue platform that sets the foundation for secondary procedures.
Fibrin glue (Ethicon, Sommerville, NJ) or thrombin spray (GenTrac, Middleton, WI) is applied. A drain may be placed prior to closure of the skin flaps as necessary. The incisions are dressed with antibiotic ointment.
Secondary Procedures by Region
MFNs affecting the eyelids, nose, ears or lips (type IIb) are common and are best managed with primary debulking and secondary operations. Secondary procedures should be performed at least 3-6 months after primary tumor debulking to allow for scar maturation and stabilization of skin ptosis.
Many secondary procedures are described in the literature. We provide a descriptive list organized by region.
MFNs of the Orbitotemporal Region
MFNs most commonly involve the orbitotemporal region [9]. The goal of orbitotemporal MFN surgery is to create an unobstructed visual axis with aesthetics being a secondary, but notable, consideration [23].
Lee et al. ’04 classified MFN orbit deformities as: brow ptosis, upper lid infiltration with ptosis, lower lid infiltration, lateral canthal disinsertion and conjunctival and lacrimal gland infiltration. Operations to address these deformities include lid and brow debulking, levator resection and suspension, frontalis sling with fascia lata grafts, lateral canthal reattachment with direct suturing, periostal flaps, static fascia lata grafts, and various other eyelid reconstructions with local flaps [7,18,20-22,24-30].
Jackson et al.’93 classified orbitotemporal neurofibromatosis as (1) with soft tissue involvement with a seeing eye, (2) bony involvement with a Seeing Eye, and (3) bony involvement with a blind, malpositioned eye [10].
Management of the seeing eye is conservative and often addressed by enlarging the orbit with osteotomies rather than debulking the intraorbital tumor, placing the orbital contents at risk [17,31].
Enucleation of the blind eye is occasionally necessary [17]. Orbital implants or free flap reconstruction is usually not necessary, as there is often enough tissue to fill the vacant orbit with local flaps [32,33,34].
In children, indications for urgent surgery for blepharoptosis include compromised visual axis and limiting amblyopia. Otherwise, most agree that aesthetic surgery in children should be delayed until after stabilization of the tumor, usually around age 18 [35,36].
MFNs of the Nasolabial Region
MFN involvement of the midface is less common than of the orbitotemporal region. The midface includes the nasolabial and oral facial units. Singal et al. ’14 recognizes two distinct mechanisms of midface distortion, each treated differently. He describes (1) primary MFN infiltration and (2) mass effect from gravitational pull of the involved cheek. Consistent with our treatment algorithm, he recommends first elimination of the lateral cheek mass, or primary debulking, with secondary attention to the midline subunits.
Primary tumor invasion of the nasolabial region is divided into three categories: (1) Involvement of the upper nose (2) involvement of the lower nose (3) total nasal involvement. For category one, only partial excisions were performed, likely because of the bony support present in this zone. For lower nasal infiltration, cartilage repositioning was performed in half of the cases. For total nasal involvement, radical excision was performed with a forehead flap being the most common reconstructive method [29,37].
Cheek MFNs predominantly cause deformity to the less stable lower half of the nose with inferolateral malpositioning of the alar subunit. Singal et al. ’14 recognized this common deformity, but offered no particular reconstructive method [37]. In our experience, suture suspension is a reliable method for alar subunit repositioning and can be performed during the primary debulking procedure.
MFNs of the Lips
Singal et al. ’14 offered similar recommendations for treatment of the lips. For primary lip infiltration, tumor excision was performed through a nasolabial fold or medially based Weber-Ferguson incision. Suture or fascia lata graft suspension was then performed, connecting the oral commissure to the orbital rim. These procedures can be performed during the primary or secondary operations [37].
For the deformed lip due to gravitational pull of a cheek MFN, nasolabial excision, fascia lata graft or suture suspension was employed. In the more complex cases (see types IIIb, IIIc below), double free flap reconstruction was performed using anterolateral thigh flaps for bulk and neurotized free gracilis flaps for animation [37].
MFNs of the Ear
Only one case report was found describing management of total ear loss with MFN resection. In this study, a prosthetic ear was utilized with good results [38]. We recommend this conservative management, as autologous reconstruction or the use of bioengineered frameworks would be fraught with complications due to soft tissue shift, postoperative scarring, and infiltration of the temporal fascia with tumor.
MFNs of the Scalp
Massive neurofibromas of the scalp are often treated by embolization, primary resection, and skin grafting. Local flaps often cannot correct the resultant alopecia, and tissue expansion is performed for hair coverage [39,40].
Studies show that neurofibromas compromise the quality of the surrounding normal skin and increase tissue expansion complications. However, scalp and forehead skin seem to be resistant to the deleterious effects of these tumors, and can be expanded successfully [41].
Skin Quality: Class III
The third and final tier of the classification system focuses on the quality of skin overlying the tumor and the general craniofacial structure. Patients with sufficient satisfactory skin quality overlying their tumors are classified as IIIa. These patients’ wounds can be reconstructed with local skin flaps. The surgeon should be comfortable using the skin overlying the tumor for reconstruction, as studies have shown good scar results using local skin flaps [42].
Patients with poor skin quality overlying their tumors, but with acceptable structural integrity of the face, are considered class IIIb. The post-resection defects of these patients do not require bulky tissue to obtain symmetry. Techniques to obtain soft tissue coverage for these wounds include local or regional skin flaps [43,44], tissue expansion (when the scalp is involved) [12,39-41], skin grafts [29], and thin fasciocutaneous free flaps [33].
In the most severe form of the disease, classified as type IIIc, patients have poor skin quality overlying their MFNs and grossly distorted facial structure. In this situation, free tissue transfer, or a face transplant, is appropriate [10]. Free flaps should be designed to replace the necessary missing structures. Fasciocutaneous or musculocutaneous flaps may be used for bulk to obtain symmetry after radical resections, while osteocutaneous free flaps may be used to obtain skeletal support [12]. Free functional flaps may be used when the face is paralyzed by the tumor or due to iatrogenic causes. In these cases, it is common to perform a double free flap. For example, an anterior lateral thigh flap for skin coverage and a free functional gracilis flap for reanimation [32,37].
Importantly, if surgical planning includes preoperative embolization and a free flap, the surgeon and interventional radiologist must discuss preservation of recipient (anastomosing) vessels.
The vascularized composite allograft, or facial transplant, is a progressing option for the patient with the most severe deficits. These patients must have emotional, institutional, and financial support. This method of treatment may provide patients suffering from diffuse, severe MFNs the opportunity to significantly improve facial function and aesthetics, as well as quality of life, despite the current cost of treatment, high dose immunosuppressant requirements, persistent threat of rejection, infection, malignancy, and high surgical and anesthesia risks [12,45-51].
Complications
The major complication of MFN surgery is massive, uncontrollable bleeding with blood product requirements of up to 40 units described in the literature [3,15,38]. Bleeding control strategies are not included in our treatment algorithm, as the surgical team should prepare for bleeding in all cases.
Preoperative CT angiography is recommended to appreciate the vascularity and location of feeding vessels [3]. Arterial embolization carries risk. In our opinion, the benefits outweigh the risks, and we recommend this procedure as indicated. The literature is inconsistent regarding the efficacy of preoperative embolization [10,15,33,39,52], but in our experience, this procedure significantly reduces bleeding.
Blood and blood products should be ordered, and autologous banked blood should be considered, especially for patients with certain religious beliefs. A cell saver autologous blood recovery system (Haemonetics, Braintree, MA) may also be prepared. Blood pressure should be kept low throughout the case, with the use of hypotensive anesthesia. Hemostatic instruments and medications such as the Ligasure small jaw open instrument (Medtronic, Minneapolis, MN), PEAK PlasmaBlade (Medtronic, Minneapolis, MN), fibrin glue (Ethicon, Sommerville, NJ) or thrombin spray (GenTrac, Middleton, WI) are invaluable tools for the modern surgeon performing MFN resection [7,15]. With massive, uncontrolled bleeding, the argon coagulator (ERBE, USA, Inc. Marietta, GA) may be used; the wound is left open and packed with surgicel (Ethicon, Cincinnati, OH), and the patient returned to the OR in 48 hours [10]. The benefits of modern surgical instruments cannot be understated.
Risks of seroma, hematoma, scarring, and infection are reduced using the common Perioperative preparation and surgical techniques.
Postoperative Management
In the immediate postoperative period, the level of care depends on blood loss. Patients requiring multiple transfusions should be placed in step down units, while patients requiring massive transfusion protocols should be placed in the intensive care unit for monitoring until stable.
Postoperatively, pain is controlled to keep blood pressure from excessive elevation, the patient’s head is kept elevated and strenuous activities are restricted. Steroids may be considered for edema, swelling and pain. After discharge, regular follow up is recommended due to the risk of recurrence and malignant transformation, as well as scar and skin ptosis observation.
Conclusion
MFNs are rare tumors posing medical, surgical reconstructive, functional, and aesthetic medical challenges. The tumors have a propensity for recurrence and a moderate rate of malignant transformation. We believe that our treatment algorithm and classification system, based on the most up to date literature, provides surgeons with practical treatment guidelines for efficient and safe preoperative planning.
The first step in preoperative planning is to determine whether bone is not (Ia) or is (Ib) involved in the tumor so that an appropriate surgical collaboration with the proper specialists and planning as a team may be implemented. A stable hard and soft tissue foundation is of utmost importance for lasting functional and aesthetic results.
The protection or restoration of facial function is the most important determinant of successful surgery, with aesthetics a noteworthy second. MFNs not involving the special structures (IIa) may be treated with a single ablative operation, while MFNs involving the special structures (IIb) will require staged surgeries for optimal outcomes.
The final consideration is wound healing, facial contour, and aesthetics. For the patient with good skin quality overlying the MFN (IIIa), local skin flap reconstruction is performed. For the patient with poor tumor skin quality but with intact underlying facial structures (IIIb), skin grafting, tissue expansion, dermabrasion, or free flaps can be considered. Currently, for the patient with poor tumor skin quality and obliterated facial structure (IIIc), free flaps provide the best reconstructive outcome. Reconstruction with vascularized composite allograft is an experimental option that may prove valuable in the future.
All MFNs share risks for recurrence, malignant transformation, and uncontrollable bleeding during surgery. All patients will benefit from regular follow-up for surveillance for recurrence and rapid growth suggestive of malignancy.
All patients should undergo CT, MRI and possible preoperative selective arterial embolization for bleeding risk stratification and control. On the day of surgery, large quantities of blood products should be made available. Hemostatic instruments and medications are essential and drastically improve the safety profile of these operations.
Our new classification system and treatment algorithm provides surgeons with a guide for preoperative planning to improve outcomes in the treatment of MNFs.
References
- Fijalkowska M, Antoszewski B. Treatment of patients with giant tumors in the course of Recklinghausen disease - own experience. Pol Przegl Chir. 2012; 84: 632-637.
- Huang W, Chong WS. Facial plexiform neurofibroma: is it truly just skin deep? BMJ Case Rep. 2013.
- Fadda MT, Giustini SS, Verdino GG, et al. Role of maxillofacial surgery in patients with neurofibromatosis type I. The Journal of craniofacial surgery. 2007; 18: 489-496.
- Neville HL, Seymour-Dempsey K, Slopis J, et al. The role of surgery in children with neurofibromatosis. Journal of pediatric surgery. 2001; 36: 25-29.
- Maceri DR, Saxon KG. Neurofibromatosis of the head and neck. Head & neck surgery. 1984; 6: 842-850.
- Lu-Emerson C, Plotkin SR. The Neurofibromatoses. Part 1: NF1. Reviews in neurological diseases. 2009; 6: E47-53.
- Lee V, Ragge NK, Collin JR. Orbitotemporal neurofibromatosis. Clinical features and surgical management. Ophthalmology. 2004; 111: 382-388.
- Kotamarti VS, Feintisch AM, Datiashvili RO. Large Neurofibroma of the Face. Eplasty. 2015; 15: ic36.
- Jacquemin C, Bosley TM, Svedberg H. Orbit deformities in craniofacial neurofibromatosis type 1. AJNR. American journal of neuroradiology. 2003; 24: 1678-1682.
- Jackson IT. Neurofibromatosis of the skull base. Clinics in plastic surgery. 1995; 22: 513-530.
- Jeblaoui Y, Neji B, Haddad S, Mnif D, Hchicha S. [Difficulties of the management of head and neck neurofibromatosis]. Annales de chirurgie plastique et esthetique. 2007; 52: 43-50.
- Janes LE, Sabino J, Matthews JA, Papadimitriou JC, Strome SE, Singh DP. Surgical management of craniofacial neurofibromatosis type 1 associated tumors. Journal of Craniofacial Surgery. 2013; 24: 1273-1277.
- Nagata S. A systematic multiple stage surgical approach for attainment of satisfactory and favourable surgical results in an extremely severe von Recklinghausen's disease, elephantiasis neurofibromatosa. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2006; 59: 662-674.
- Chiumariello S, Del Torto G, Guarro G, Alfano C. The role of plastic surgeon in complex cephalic malformations. Our experience. Ann Ital Chir. 2014; 85: 166-170.
- Latham K, Buchanan EP, Suver D, Gruss JS. Neurofibromatosis of the head and neck: classification and surgical management. Plastic & Reconstructive Surgery. 2015; 135: 845-855.
- Krastinova-Lolov D, Hamza F. The surgical management of cranio-orbital neurofibromatosis. Annals of plastic surgery. 1996; 36: 263-269.
- Jackson IT, Carbonnel A, Potparic Z, Shaw K. Orbitotemporal neurofibromatosis: classification and treatment. Plastic and reconstructive surgery. 1993; 92: 1-11.
- Pessis R, Lantieri L, Britto JA, Leguerinel C, Wolkenstein P, Hivelin M. Surgical care burden in orbito-temporal neurofibromatosis: Multiple procedures and surgical care duration analysis in 47 consecutive adult patients. Journal of cranio-maxillo-facial surgery: official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2015; 43: 1684-1693.
- Chen JY, Muecke JS, Brown SD. Orbital plexiform neurofibroma and high axial myopia. Ophthalmic plastic and reconstructive surgery. 2008; 24: 284-286.
- Chaudhry IA, Morales J, Shamsi FA, et al. Orbitofacial neurofibromatosis: clinical characteristics and treatment outcome. Eye. 2012; 26: 583-592.
- Singhal D, Chen YC, Chen YR, Chen PK, Tsai YJ. Soft tissue management of orbitotemporal neurofibromatosis. The Journal of craniofacial surgery. 2013; 24: 269-272.
- Van der Meulen J. Orbital neurofibromatosis. Clinics in plastic surgery. 1987; 14: 123-135.
- Altan-Yaycioglu R, Hintschich C. Clinical features and surgical management of orbitotemporal neurofibromatosis: a retrospective interventional case series. Orbit (Amsterdam, Netherlands). 2010; 29: 232-238.
- Lind J, Walker G, Verheyden CN. Restoration of normal eyelid function after resection of orbitotemporal neurofibroma. Plastic and reconstructive surgery. 2011; 128: 74e-75e.
- Coban-Karatas M, Altan-Yaycioglu R, Bal N, Akova YA. Management of facial disfigurement in orbitotemporal neurofibromatosis. Ophthalmic plastic and reconstructive surgery. 2010; 26: 124-126.
- Li J, Lin M, Shao C, Ge S, Fan X. Blepharoplasty techniques in the management of orbito-temporal neurofibromatosis. Journal of Plastic, Reconstructive & Aesthetic Surgery: JPRAS. 2014; 67: 1496-1501.
- Morax S, Herdan ML, Hurbli T. The surgical management of orbitopalpebral neurofibromatosis. Ophthalmic plastic and reconstructive surgery. 1988; 4: 203-213.
- Marchac D, Britto JA. Remodelling the upper eyelid in the management of orbitopalpebral neurofibromatosis. British journal of plastic surgery. 2005; 58: 944-956.
- Chen YR, Chen KT, Noordhoff MS. Facial elephantiasis neurofibromatosa--excision and skin graft. Annals of plastic surgery. 1989; 23: 547-551.
- Fan XQ, Lin M, Li J, Zhou HF, Fu Y. [Removal and plastic reconstructive surgery of orbital neurofibroma]. Zhonghua yi xue za zhi. 2007; 87: 3264-3267.
- Marchac D. Intracranial enlargement of the orbital cavity and palpebral remodeling for orbitopalpebral neurofibromatosis. Plastic and reconstructive surgery. 1984; 73: 534-543.
- Uygur F, Chang DW, Crosby MA, Skoracki RJ, Robb GL. Free flap reconstruction of extensive defects following resection of large neurofibromatosis. Annals of plastic surgery. 2011; 67: 376-381.
- Singhal D, Chen YC, Fanzio PM, et al. Role of free flaps in the management of craniofacial neurofibromatosis: soft tissue coverage and attempted facial reanimation. J Oral Maxillofac Surg. 2012; 70: 2916-2922.
- Madill KE, Brammar R, Leatherbarrow B. A novel approach to the management of severe facial disfigurement in neurofibromatosis type 1. Ophthalmic plastic and reconstructive surgery. 2007; 23: 227-228.
- Lee V, Ragge NK, Collin JR. The surgical management of childhood orbito-temporal neurofibromatosis. British journal of plastic surgery. 2003; 56: 380-387.
- Acarturk TO, Yigenoglu B, Pekedis O. Excision and "transcutaneous" lift in patients with neurofibromatosis of the fronto-temporo-orbital and auricular regions. The Journal of craniofacial surgery. 2009; 20: 771-774.
- Singhal D, Chen YC, Tsai YJ, et al. Craniofacial neurofibromatosis: treatment of the midface deformity. Journal of cranio-maxillo-facial surgery: official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2014; 42: 595-600.
- Cheng XB, Lei DL, Li YP, et al. Surgical treatment of a giant neurofibroma. Journal of Craniofacial Surgery. 2011; 22: 2244-2246.
- Yuan SM, Guo Y, Cui L, et al. Surgical Management of Giant Scalp Neurofibroma after Ultra-Selective Embolization of Nutrient Artery. Journal of Craniofacial Surgery. 2015; 26: e405-407.
- Simsek ME, Kahveci R. Huge neurofibroma of the scalp. Indian J Med Res. 2014; 140: 695.
- Singhal D, Chen YC, Seselgyte R, Chen PK, Chen YR. Craniofacial neurofibromatosis and tissue expansion: long-term results. Journal of Plastic, Reconstructive & Aesthetic Surgery: JPRAS. 2012; 65: 956-959.
- Miyawaki T, Billings B, Har-Shai Y, et al. Multicenter study of wound healing in neurofibromatosis and neurofibroma. The Journal of craniofacial surgery. 2007; 18: 1008-1011.
- Hanke CW, Conner AC, Reed JC. Treatment of multiple facial neurofibromas with dermabrasion. The Journal of dermatologic surgery and oncology. 1987; 13: 631-637.
- Karabekmez FE, Duymaz A, Karacor Z. Dermabrasion and staged excision of facial lesions in a neurofibromatosis case for improvement of facial appearance. Journal of Cutaneous Medicine & Surgery. 2013; 17: 362-364.
- Sicilia-Castro D, Gomez-Cia T, Infante-Cossio P, et al. Reconstruction of a severe facial defect by allotransplantation in neurofibromatosis type 1: a case report. Transplantation proceedings. 2011; 43: 2831-2837.
- Sedaghati-nia A, Gilton A, Liger C, et al. Anaesthesia and intensive care management of face transplantation. British journal of anaesthesia. 2013; 111: 600-606.
- Gomez-Cia T, Sicilia-Castro D, Infante-Cossio P, et al. Second human facial allotransplantation to restore a severe defect following radical resection of bilateral massive plexiform neurofibromas. Plastic and reconstructive surgery. 2011; 127: 995-996.
- Siemionow M, Ozturk C. An update on facial transplantation cases performed between 2005 and 2010. Plastic and reconstructive surgery. 2011; 128: 707e-720e.
- Shanmugarajah K, Hettiaratchy S, Clarke A, Butler PE. Clinical outcomes of facial transplantation: a review. International journal of surgery (London, England). 2011; 9: 600-607.
- Ruegg EM, Hivelin M, Hemery F, et al. Face transplantation program in France: a cost analysis of five patients. Transplantation. 2012; 93: 1166-1172.
- Gordon CR, Siemionow M, Papay F, et al. The world's experience with facial transplantation: what have we learned thus far? Annals of plastic surgery. 2009; 63: 572-578.
- Tomei KL, Gupta V, Prestigiacomo CJ, Gandhi CD. Spontaneous hemorrhage of a facial neurofibroma: endovascular embolization before surgical intervention. Journal of Craniofacial Surgery. 2013; 24: e514-517.