Research Article
Ann Surg Perioper Care. 2016; 1(2): 1014.
Enterocutaneous Fistula: Guidelines for an Evolving Problem
Cheaito A*, Tillou A, Lewis C and Cryer H
Assistant Professor of Surgery, University of California, David Geffen School of M edicine, Los Angeles, USA
*Corresponding author: Ali Cheaito, Assistant Professor of Surgery, University of California, David Geffen School of Medicine, 10833 Le Conte Ave, 72-232 CHS, Los Angeles, USA
Received: November 11, 2016; Accepted: December 06, 2016; Published: December 08, 2016
Abstract
Importance: The care and outcome of enterocutaneous fistula (ECF) have improved greatly over several decades due to revolutionary advances in nutrition, along with dramatic improvements in the treatment of sepsis in the critically ill. However, as the collective experience with damage control surgery has matured, the frequent development of enteroatmospheric fistulas (EAF) in the open abdomen patient has emerged as an even more vexing problem. Despite our best efforts, ECFs and especially EAFs continue to be highly morbid conditions, and sepsis and malnutrition remain the leading causes of death. Aggressive nutritional, metabolic support and multidisciplinary approach is the most significant predictor of outcome with ECFs and EAFs.
Observations: Discussion of the historical advances in therapy and their impact on ECFs, as well as review of the classification of ECFs and EAFs, provides a framework for the suggested phased strategy that specifically targets the nutritional and metabolic needs of the ECF/EAF patient. These three phases include (1) diagnosis, resuscitation, and early interval nutrition; (2) definition of fistula anatomy, drainage of collections, nutritional assessment and monitoring, and placement of feeding access; and (3) definitive operative management.
Conclusion: The successful management of GI fistula requires a multidisciplinary team approach including a gastroenterologist, interventional radiologist, enterostomal therapist, dietician, social worker and surgeons. With this coordinated approach, EC fistula can be controlled with acceptable morbidity and mortality.
Keywords: Enterocutaneous fistula; Enteroatmospheric fistulas; Gastrointestinal tract
Introduction
An Enterocutaneous Fistula (ECF) is defined as an abnormal connection between the gastrointestinal tract and the skin, and requires labor-intensive medical management and surgical expertise. ECFs are increasing in prevalence and are characterized by difficult management and healing with a mortality rate ranging between 6% and 33% [1,2,3]. Complex wound care, severe malnutrition, frequent infectious complications, chronic pain, and depression require significant investment of health care resources and make the shortterm and longterm care of these patients difficult. The incidence of ECFs depends on the underlying abdominal pathology and varies between 2% and 25% for trauma patients, 20% and 25% for abdominal sepsis, and up to 50% for infected pancreatic necrosis [4-7]. Previous studies have shown that ECFs occur as a result of a variety of factors with surgical misadventure being the most common (Figure 1). Other factors include malignancy, inflammatory bowel disease, post radiation therapy for malignancy, distal obstruction, iatrogenic or spontaneous bowel injury, complicated intra-abdominal infections such as tuberculosis, amoebiasis, and typhoid, or diverticular disease.
The first major series on ECFs emerged from Massachusetts General Hospital in 1960 and reported a staggering 44% mortality rate [8,9]. Recent advances in nutritional and metabolic support, wound care, interventional radiology, and surgical technique have resulted in an overall decline in mortality to 5% to 15% [10]. ECFs can be classified based on a number of characteristics including complexity, output volume, anatomic location, and cause (Table 1). Low output is classified as < 200cc/day, moderate output as 200-500cc/day, and high output as > 500cc/day. Complexity is rated on a scale of one to four with one being simple and four being the most complex. EAF fistula is defined as an exposed fistula occurring in the midst of an open abdomen with no overlying soft tissue. By nature, complex fistulas have higher morbidity and mortality rates, as well as a lower rate of spontaneous closure. EAFs usually develop as a consequence of one or more of the following factors: postoperative anastomotic disruption, deserosalizations occurring during laparotomy, exposure of dehydrated and desiccated bowel to several materials used for temporary abdominal closure, adhesions between the edematous bowel and the anterior abdominal wall, severe wound infections, burst abdomen, severe trauma, sepsis with known precipitating factors, and finally, preceding bowel ischemia.
Fistula Classifications
Description
ECF category
I: Single orifice passing through flat area of skin that is in good condition
II: Single or multiple ECF orifices close to bony prominences, surgical scars, other stomas, or the umbilicus
III: ECF presenting through small dehiscence within a single main wound
IV: ECF at the base of gaping wound or large dehiscence
Daily output
Low: <200ml
Intermediate: 200-500ml
High: >500ml
Anatomical Location
Gastric, small bowel, color, or rectum
Cause
Iatrogenic, mesh, IBD, trauma, radiation, or neoplasm
Table 1: Classification of ECF.
Unfortunately, nonoperative closure rates continue to remain low at 5% to 20%, and definitive operative closure is successful only 75% to 85% of the time [11,12]. Randomized studies regarding surgical management of ECF are nonexistent, and most accepted standards are based on expert opinion. The operative management often requires lengthy procedures in hostile abdomens with abundant adhesions and surrounding inflammation. In addition to the significant risk of mortality, morbidity can be equally as devastating. Even after a fistula is closed, the probability for recurrence is relatively high and seen in 9%–33% of spontaneously sealed ECF/EAF patients [10,13].
Fistula location, demonstration of other intra-abdominal abscesses or associated fluid collections, and exclusion of distal gastrointestinal (GI) obstruction can be evaluated for by a wide variety of imaging diagnostic methods including: methylene blue test, upper and lower GI series with water soluble contrasts, fistulography, computed tomography, and magnetic resonance imaging.
Tertiary and quaternary medical centers are seeing a surge in patients transferred for management of ECF/EAF. This review is intended to describe the principles of management of ECF.
Methods
The PubMed database was searched for all human subject, English language articles published during the past 20 years (January 1, 2000
- January1, 2015) using the search term enterocutaneous fistula/ enteroatmospheric fistula, resulting in 568 matches. All articles were screened for inclusion via an assessment of the title for appropriateness. We then filtered the remaining 118 articles by reviewing the abstracts. Review articles and case reports were excluded in an effort to highlight original research. Studies concerning pediatric patient were excluded. The majority of studies were retrospective in design; no randomized clinical trials were identified. Preference was given for meta-analyses, systematic reviews, and prospective trials with higher order evidence. A total of 37 articles were included in this review.
Result
ECF care protocol starts with management of skin and sepsis, nutrition, definition of fistula anatomy, and proposing a procedure to address the fistula. Many authors have suggested various stepwise systems and protocols for treating ECF (Table 2,3). In addition, it is important that the patient is treated in a center with significant experience in treating ECFs and that multidisciplinary approach is used. This later measure results in 50% decrease in mortality. The different components of ECF cares are listed as follows in the order of immediacy to the patient.
Author
Number of patients
Surgical closure
Spontaneous closure
Overall mortality
Stiges-Serra et al.
75
13.3
65.3
21.3
Conter et al.
51
80.4
9.8
7.8
Levy et al.
33.5
37.3
29
33.7
Schein and Decker
117
NA
NA
37
Chamberlain et al.
25
24
32
40
Hollington et al.
277
43.7
25.6
15.2
Table 2: Recent Studies on Management of ECF.
Favorable
Unfavorable
Surgical etiology
Ileal, Jejunal, non-surgical etiology
Appendicitis, Diverticulitis
IBD, cancer, radiation
Transferin >200mg/dl
Transferin <200 mg/dl
No obstruction, bowel in continuity, no infection, no inflamed bowel
Distal obstruction, bowel discontinuity, adjacent infection, active inflammation
Length >2cm, end fistula
Length <2cm, lateral fistula, multiple fistulas
Output <200ml/24hr
Output >500ml/24hr
No sepsis, balanced electrolytes
Sepsis, electrolytes disturbances
Initial referral to tertiary care center and subspeciality care
Delay getting to tertiary care center and subspeciality care
Table 3: Description of favorability of closure of ECF.
Initial Resuscitation and Electrolyte Repletion
Patients with ECF often suffer from electrolyte and fluid imbalances. They usually present the emergency department with clinical picture of dehydration and require hospitalization. Fluid and electrolyte losses should be replaced with crystalloids. Those patients with severe dehydration and electrolyte disturbances will require serum testing of renal function and electrolytes regularly to ensure the supplementation is progressing appropriately. One method that can be used to deduce the composition of the best replacement fluid composition is to measure electrolyte concentration in fistula effluent and to match the electrolyte composition closely to the replacement fluid (Table 4). Adequate intravenous access is mandatory to care for patients with dehydration and high-output fistulae.
NA
K
H
CL
HCO3
Chyme (stomach)
40-65
10
90
100-140
Pancreatic
135-155
5
55-75
70-90
Bile
135-155
5
80-110
35-50
Succus(small bowel)
120-130
10
50-60
50-70
Stool(colon)
25-50
35-60
20-40
30-45
Table 4: Electrolyte composition of various GI fluid.
Treatment of Sepsis
Sepsis is responsible for 77% of mortality associated with ECF [14,15]. Computed tomography of the abdomen and pelvis along with percutaneous drainage with radiographic guidance is essential to evaluate and treat sources of infection. Computed tomography has an accuracy of more than 97% when enhancing contrast media are used appropriately [16]. Radiologically guided drainage provides the fastest and safest route to evacuate and control significant infection. In addition, much information can be gained about fistula anatomy and the enteric source from a fistulogram, and percutaneous drainage may decompress a complex fistula and convert it to a simple one. In cases of peritonitis and without the ability to obtain source control with more conservative means, prompt fluid resuscitation, antibiotic administration, and operative control of infection are essential. There is no role for antibiotic coverage in a patient with ECF whose sepsis is fully controlled with percutaneous drainage. Operative sepsis control should focus on infection drainage and exteriorization of the source in the small or large intestine. Support of organ system functions and utilization of the intensive unit care are often necessary. Resolution of sepsis is mandatory in order for ECF to close spontaneously. In the state of increased catabolism, malnutrition is a predictable outcome, as is immunosuppression. Even in the absence of overt sepsis, 50% of patients with ECFs harbor intra-abdominal abscesses, most of which are amenable to percutaneous drainage [17-20].
Nutrition
Nutrition is one of the necessities upon which the life and successful treatment of a patient with ECF hinges. Fazio et al showed that mortality is 0.5% when serum albumin is > 3.5mg/dL [16]. For most patients, a combination of enteral (EN) and parental (PN) will be employed, at least initially. Fistula closure rates are twice as high in those receiving adequate supplemental nutrition as opposed to those who are not1 [21,22]. The goal of successful nutrition management is achieving an anabolic state with weight gain, improvement in albumin, prealbumin, and transferrin, and successful management of micronutrient needs for optimal healing (Table 5).
Calorie requirement (kcal/kg/d)
Protein requirement (g/kg/d)
Vitamin C
Other Vitamins
Elements
Low output
20-30
1-1.5
5-10 times normal
At least normal
At least normal
High output
25-35
1.5-2.5
10 times normal
2 times normal
2 times normal
Table 5: Requirements based on fistula output.
Several factors influenced the selection of rout of feeding, including origin of fistula, length of healthy bowel available for absorption, and fistula output. If there was sufficient functioning bowel for adequate nutrients absorption and no intra-abdominal sepsis and manageable fistula output, the enteral feeding should be the optimum choice. If the nutritional requirement could not be achieved enterally or the fistula output was high, the parenteral feeding should be used. The nutrition management usually began with TPN in the resuscitation phase. A short period of TPN feeding is preferable to avoid the disadvantages and complications related to it (Table 6), especially when administered by central line. Carbohydrate, fat, and protein calories ratio in TPN could be modified according to patient’s medical history. TPN was used for the first time in ECF patients, in a large trial (Polk et al) that included 300 adult patients with variety of diseases that prevent feeding via the gut [19]. They provided patients intravenously different amounts of calories for different periods of feeding. They reported an increase in body weight, positive nitrogen balance, persistent and spontaneous fistula closures, and noticeable decreases in the mortality rate. These findings were supported by MacFadyen et al., who presented exciting results; decreasing the mortality rate to 6.45%, and spontaneous fistula closure to 70% among the study subjects [17]. These preferable results were due to the ability of TPN to decrease gastrointestinal secretions by 30–50%, which made surgeons believe that TPN was the key in managing proximal and high output fistula. In (2003) Li et al. published their 30 years of experience in treating 1168 ECF patients in China [18]. They reported the preferable outcome was by using a combination of TPN and enteral feeding in patients with ECF. unless it is contraindicated, due to its relatively lower cost, greater availability and fewer complications. Early enteral nutrition in ECF fistula has been associated with earlier fistula closure, lower pneumonia rate and lower rate of fistula recurrence. The contraindications of enteral feeding include intestinal discontinuity, ileus, short bowel length (shorter than required 75cm of small bowel for successful enteral feeding), not achievable enteral access, or not tolerated enteral feeding. The increase in fistula output was an ECF additional contraindication for enteral feedings. The use of enteral nutrition in high output fistula is found useless, and didn’t provide any benefit to the patients, and had compounded metabolic and management complications.
Liver Disease
Steatosis, cholestasis, fibrosis, cirrhosis, liver failure, biliary sludge
Sepsis
Loss of vascular access
Thrombosis, line dislodgment
Metabolic complications
Fluid and electrolyte imbalance, acidosis, micronutrients deficiencies
Metabolic bone disease
Osteomalacia, osteopenia, osteoporosis
Renal dysfunction
Hyperoxaluria, nephrolithiasis, renal insufficiency
Neurologic
Psychological
Table 6: Complications related to TPN use.
Definition of Fistula Anatomy
Defining fistula anatomy is essential to further planning of operative repair, an optimal nutrition strategy, and patient counseling. Often, a combination of studies is necessary to fully appreciate the fistula anatomy. Computed tomography, fistulography, a small bowel follow-through study, and contrast enemas are all useful modalities for defining anatomy depending on the location of the fistula. Magnetic resonance enterography is another adjunct, particularly useful in patients with IBD. These studies should only be undertaken at least 7 to 10 days after fluid and electrolyte resuscitation, infection control, and appropriate wound treatment cares.
Management of Fistula Output
At initial assessment oral intake should be minimized to facilitate estimation of both fistula and stoma output. Losses need to be replaced intravenously with concomitant correction of serum electrolyte imbalances. Oral intake is reintroduced and included both high-caloric nutritional drinks. Supplementary nasogastric feeding is instituted where necessary. When the combined ECF and fecal output is greater than 1.5L/day, care is made to minimize losses in order to discontinue supplementary intravenous fluid rehydration. Intake of fluids low in sodium should be limited to 0.5L/day when necessary; however, patients are also advised to that an unlimited intake of electrolyte solutions containing high concentrations of sodium and glucose was acceptable. Intake of significant volumes of fluid with meals is also discouraged to minimize ECF and stoma output. The antimotility agents loperamide and codeine phosphate need to be utilised to decrease ECF output when the above measures were not successful, as were proton pump inhibitors which helped to minimize gastric secretions. Additionally Octreotide can be used when output remained high to decrease intestinal and pancreatic secretions (Figure 1).
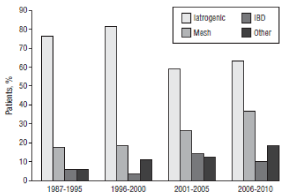
Figure 1: Distribution of etiology of ECF.
Timing of Surgery
The most essential question is this: when do we ‘‘attack’’ a frozen abdomen and an ECF? The answer is uncertain. Surgeons have wrestled with the question of when to operate and how to succeed for years. Certain anatomic factors are known to affect the closure, such as the length of the fistulous tract and the anatomic location of the fistula. Ideally, the anatomy of complex ventral hernias, ECFs, and EAFs can properly be identified (preoperatively, if at all possible). Any previous operative reports for a given patient should be obtained and studied. On many occasions, however, the surgeon must make a difficult decision and embark on operation even without completely discerning the anatomy, without having a surgical road map to follow. In such situations the surgeon can only hope that, intraoperatively, the anatomy will become clear. Overall, there are two camps of surgeons: those who wait until things have ‘‘settled down’’ and those who choose to operate early. The decision is clinical, based on the individual patient. One recent study that looked at early planned operations in patients with ECF reported a 21% mortality rate from multiple-organ system failure and a 17% complication rate [19]. But other recently reported mortality rates are as low as 7%, much better than the historical mortality rate of 43% reported in 1960 [14]. Some authors have suggested waiting 4–5 weeks before operating, just long enough to make sure that patients are nutritionally sound and that sepsis is controlled [14,15,18]. Most surgeons, however, wait 3–6 months and others wait 12 months or longer. In preparation for the operation, key clinical and laboratory issues must be addressed. For example, blood sugar levels must be controlled. Patients must stop smoking for at least a month before the surgery. The bioburden must be reduced through application of vacuum-assisted closure (VAC) of the wound or through other modalities, including stoma protection techniques. Hypovolemia and chronic anemia must be corrected. A complete biochemical profile (including levels of trace elements, vitamins, and essential fatty acids) must be obtained and any problems resolved.
Surgical intervention for patients with ECF that failed to close spontaneously was performed a minimum of 6 months after their most recent laparotomy, or if the fistulous disease did not occur as a result of surgery, at least 6 months after the ECF had arisen. This period was used to allow fistula maturation, resolution of inflammation and sepsis and return of the physiological and nutritional status of the patient, as close as possible, to their pre-morbid state. Furthermore, it has been suggested that the abdominal environment is less “hostile” with less in duration after this time period.
Surgical Procedure to Repair the Fistulous Disease
Either open or laparoscopic surgical techniques can be used to repair abdominal wall defects. But in patients with a frozen or a hostile abdomen and with ECFs and/or EAFs, the open approach is standard. Each surgeon will use creativity and a combination of different techniques and repairs, depending, primarily, on the mission at hand. If the goals of the operation are to take down fistulas, establish GI tract continuity, and concomitantly repair abdominal defects, then things become more complicated, and the surgeon should plan accordingly. Preoperatively, surgeons should assume that all patients with ECFs and/or EAFs have a frozen abdomen or a hostile abdomen, and that entering the abdominal cavity will be extremely challenging. Proper mental preparation is essential for the surgeon as well as for the patient and family. When possible, the surgeon should avoid going through the same incision used in prior operations. Instead, attempts should be made to enter the abdomen from non-violated areas of the abdominal wall. But doing so may not always be possible, especially in patients who have previously undergone laparoscopy for trauma or other major operations. Some authors have suggested alternative methods of entering the abdomen through a transverse incision. During fascial closure, utmost care must be taken to avoid injury to the underlying bowel: the consequences of inadvertent enterotomies are not trivial. In one study, patients with inadvertent enterotomies had a significantly higher rate of postoperative complications (P<0.01) and of urgent relaparotomies (P<0.001), a higher rate of admission to the ICU (P<0.001) and of parenteral nutrition use (P<0.001), and an increased postoperative hospital length of stay (P<0.001) [22]. If an enterotomy is recognized, it should be either repaired at once or marked with a silk suture for later identification. Most authors agree that surgeons should mobilize and identify the entire GI tract, from the gastroesophageal (GE) junction to the recto sigmoid junction. Identifying all of the fistulas and the entire GI tract is pivotal. Resecting multiple fistulas as one segment end masse is preferable, but this may not be possible if the fistulas are located at a distance from one another. Thus, difficult decisions must often be made during the course of the operation: Should more than two or three anastomoses be created, running the risk of a leak or an ECF? Or, should the number of anastomoses be minimized? Should large segments of small bowel be resected, potentially creating GI-crippled patients with possible short gut syndrome? Or should one create more anastomoses? Only the operating surgeon can make that judgment. It is important to recognize that intestines look shorter than they in fact are in the abdomen that has been operated previously. If at least 20–25 cm of bowel can be left between anastomoses, a hand-sewn doublelayer technique should be used. To avoid resecting a large amount of bowel, adjunct procedures (such as a modified stricture plasty) can be used in certain fistulas. If the integrity of the anastomoses or anastomosis is questionable, revision is reasonable, as is creation of a proximal diverting ostomy. Surgeons should not promise their patients that they will not have a stoma, temporary or otherwise.
Reconstruction of Abdominal Wall Defects
The goal of the operation in patients with ECFs and/or EAFs is to definitively correct the problem. Operative treatment with takedown of ECFs or fistula excision and abdominal wall reconstruction is successful in 80–90% of patients [17]. Once GI tract continuity has been established (as described above), the next big step is to cover the intraperitoneal content. But doing so can be a serious surgical challenge. To cover the abdominal cavity and create the ‘‘new’’ abdominal wall, native tissue and prosthetic mesh should be used. No one technique has been reported to be superior to others. Many authors have described a combination of different approaches, based on the location and the type of defects. Using a combination of different techniques and a preplanned algorithm based on careful analysis of the defect and location, a 92% rate of successful closure of this complex of defects has been reported [22-27].
Intraoperative Technique
Many studies indicate that operative duration, estimated blood loss, resection type, and abdominal wall closure each play a significant role in patient outcomes. One-third of all cases with the operation lasting longer than 8 hours and more than half of the cases with significant blood loss (>1000mL) were associated with a recurrent fistula and increased risk of 1-year mortality [28]. In multiple series, univariate and multivariate analyses demonstrate that ECF recurrence is more likely after over sewing the fistula instead of resection with primary anastomosis [16,22,28]. Similarly, Brenner et al report that ECF recurrence is 4 times more likely in patients with stapled anastomoses compared with hand-sewn ones [29-31]. Patients who have a primary repair of their ECF instead of a resection have a 3-fold increased risk for 1-year mortality. Primary repair of ECF should be discouraged as a definitive surgical technique for closure. Abdominal wall closure after ECF repair has a considerable effect on patient outcomes. Patients whose fascia we were unable to close had more than doubled the risk of ECF recurrence and 1-year mortality [31]. Without appropriate fascial coverage, even the best-repaired fistulas are much less likely to heal.
Postoperative Care
More than 35% of patients developed at least 1 episode of postoperative sepsis and/or shock; among these patients, one-third developed a recurrent fistula and one-fourth died within 1 year [32,33]. Martinez et al similarly reported that postoperative sepsis is the most important factor associated with mortality in patients with ECF [34]. Furthermore, patients who developed an organ space surgical site infection (eg: intra-abdominal abscess) were 4 times more likely to have refistulization. Whether the organ space surgical site infection is causative or merely an indicator of refistulization, all efforts should be made intraoperatively to minimize excessive contamination and to repair deserosalizations [35]. Patients who required mechanical ventilation for longer than 48 hours postoperatively had nearly 5 times the risk of refistulization. Moreover, patients requiring blood transfusion within 72 hours of the start of surgery had an almost 4-times greater risk of recurrent fistula. One might assume that anemia at any point during the healing process, whether during or after surgery, results in decreased oxygen delivery to a healing anastamosis, thus increasing its risk for breakdown. Postoperative ECF recurrence was associated with a 3-fold increased risk of death within the first year following ECF repair. Likewise, Brenner et al reported that ECF recurrence is a primary determinant for mortality. Thus, any efforts to prevent recurrence would also directly prevent mortality.
Conclusion
ECF can be a life-threatening condition requiring longitudinal care for many months. A spectrum of vexing clinical problems ranging from hypovolemic shock to malnutrition to complex abdominal wall reconstruction challenge the skill of even highly experienced surgeons. The management of ECF requires adherence to the basic principles of sepsis control, wound and skin care, nutrition optimization, fluid and electrolyte balance, psychological support, and case management both in the hospital and home health care. With all these various needs, the utility of a multidisciplinary approach cannot be overemphasized. Surgical treatment of patients with ECFs and a hostile abdomen and other complex abdominal defects is challenging and expensive; it requires significant resources, both surgical and financial. Careful planning and advanced surgical techniques are required, often involving the use of biologic mesh and composite tissue transfer. Careful selection of patients, aggressive preoperative management, judicious intraoperative technique, and vigilant postoperative care are essential for successful outcomes following the surgical closure of ECF. In particular, preoperative nutritional optimization, abdominal fascia closure, and prevention of postoperative complications remain paramount for preventing refistulization and postoperative mortality.
References
- Kirshtein B, Mizrahi S. Vacuum-assisted management of enteroatmospheric fistula within the open abdomen. Am Surg. 2014; 80: e209-e210.
- Tavusbay C, Genc H, Cin N, et al. Use of a vacuum-assisted closure system for the management of enteroatmospheric fistulae. Surg Today. 2014; 45.
- Schecter WP. Management of enterocutaneous fistulas. Surg Clin North Am. 2011; 91: e481-e491.
- Davis KG, Johnson EK. Controversies in the care of the enterocutaneous fistula. Surg Clin North Am. 2013; 93: e231-e250.
- Evenson A, Fischer J. Current management of enterocutaneous fistula. J Gastrointest Surg. 2006; 10: e455-e464.
- Coughlin S, Roth L, Lurati G, Faulhaber M. Somatostatin analogues for the treatment of enterocutaneous fistulas: a systematic review and metaanalysis. World J Surg. 2012; 36: e1016-e1029.
- Stevens P, Foulkes RE, Hartford-Beynon JS, Delicata RJ. Systematic review and meta-analysis of the role of somatostatin and its analogues in the treatment of enterocutaneous fistula. Eur J Gastroenterol Hepatol. 2011; 23: e912-e922.
- Rahbour G, Siddiqui MR, Ullah MR, et al. A meta-analysis of outcomes following use of somatostatin and its analogues for the management of enterocutaneous fistulas. Ann Surg. 2012; 256: e946-e954.
- Lloyd DA, Gabe SM, Windsor AC. Nutrition and management of enterocutaneous fistula. Br J Surg 2006; 93: e1045-e1055.
- Nightingale JM. The medical management of intestinal failure: methods to reduce the severity. Proc NutrSoc. 2003; 62: e703-e710.
- Marinis A, Gkiokas G, Argyra E, et al. “Enteroatmospheric fistulae” d gastrointestinal openings in the open abdomen: a review and recent proposal of a surgical technique. Scand J Surg. 2013; 102: e61-e68.
- Krpata DM, Stein SL, Eston M, et al. Outcomes of simultaneous large complex abdominal wall reconstruction andenterocutaneous fistula takedown. Am J Surg. 2013; 205: e354-e359.
- Altomare DF, Serio G, Pannarale OC, et al. Prediction of mortality by logistic regression analysis in patients with postoperative enterocutaneous fistulae. Br J Surg. 1990; 77: 450–453.
- Reinhardt GF, Myscofski JW, Wilkens DB, et al. Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. JPEN J Parenteral Enteral Nutr. 1980; 4: 357–359.
- Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999; 134: 36–42.
- Kuvshinoff BW, Brodish RJ, McFadden DW, et al. Serum transferrin as a prognostic indicator of spontaneous closure and mortality in gastrointestinal cutaneous fistulas. Ann Surg. 1993; 217: 615–622; discussion 622–623.
- Rosenthal RA. Nutritional concerns in the older surgical patient. J Am Coll Surg. 2004; 199: 785-791.
- Jernigan TW, Fabian TC, Croce MA et al. Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg. 2003; 238: 349–355; 355–357.
- Sriussadaporn S, Sriussadaporn S, Kritayakirana K, et al. Operative management of small bowel fistulae associated with open abdomen. Asian J Surg. 2006; 29: 1–7.
- Khan J, Iiboshi Y, Cui L, et al. Alanyl-glutamine supplemented parenteral nutrition increases luminal mucus gel and decreases permeability in the rat small intestine. JPEN J Parenter Enteral Nutr. 1999; 23: 24–31.
- Draus JM Jr, Huss SA, Harty NJ, Cheadle WG, Larson GM. Enterocutaneous fistula: are treatments improving? Surgery. 2006; 140: 570–576, discussion 576–578.
- Martinez JL, Luque-de-Leon E, Mier J, Blanco-Benavides R, Robledo F. Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes. World J Surg. 2008; 32: 436–443, discussion 444.
- Visschers RG, OldeDamink SW, Winkens B, Soeters PB, van Gemert WG. Treatment strategies in 135 consecutive patients with enterocutaneous fistulas. World J Surg. 2008; 32: 445–453.
- Lynch AC, Delaney CP, Senagore AJ, Connor JT, Remzi FH, Fazio VW. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004; 240: 825–831.
- Bressler B, Sands BE. Review article: medical therapy for fistulizing Crohn’s disease. Aliment Pharmacol Ther. 2006; 24: 1283–1293.
- Pieper B, Mikols C, Dawson Grant T. Comparing adjustment to an ostomy for three groups. J Wound Ostomy Continence Nurs.1996; 23: 197-204.
- Evenson A, Fischer J. Current management of enterocutaneous fistula. J Gastrointest Surg. 2006; 10: 455-464.
- Sprangers M, Taal B, Aaronson N, teVelde A. Quality of life in colo-rectal cancer. Dis Colon Rectum. 1995; 38: 361-369.
- Foulis W, Mayberry J. Elderly ileostomists and their social problems. J Clin Gastroenterol. 1990; 12: 276-268.
- Martinez JL, Luque-de-Leon E, Mier J, Blanco-Benavides R, Robledo F. Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes. World J Surg. 2008; 32: 436–443, discussion 444.
- Li J, Ren J, Zhu W, Yin L, Han J. Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J (Engl). 2003; 116: 171–175.
- Campos AC, Andrade DF, Campos GM, Matias JE, Coelho JC. A multi variate model to determine prognostic factors in gastrointestinal fistulas. J Am Coll Surg. 1999; 188: 483–490.
- Hollington P, Mawdsley J, Lim W, Gabe SM, Forbes A, Windsor AJ. An 11- year experience of enterocutaneous fistula. Br J Surg. 2004; 91: 1646–1651.
- Reber HA, Roberts C, Way LW, Dunphy JE. Management of external gastrointestinal fistulas. Ann Surg. 1978; 188: 460–467.
- Rahbour G, Gabe SM, Ullah MR, et al. Seven-year experience of enterocutaneous fistula with univariate and multivariate analysis of factors associated with healing: development of a validated scoring system. Colorectal Dis. 2013; 15: 1162–1170.