Research Article
Ann Surg Perioper Care. 2016; 1(3): 1018.
Glissonean Pedicle Transection Method using Vascular Stapling Devices in Anatomic Liver Resections: A Single Centre Experience
Karamarković A¹*, Bracanović M², Bajec A², Bumbaširević V³, Jovanović B³ and Vujadinović ST4
¹Faculty of Medicine University of Belgrade, Clinic for Emergency Surgery, Clinical Center of Serbia, Belgrade, Serbia
²Clinic for Emergency Surgery, Clinical Center of Serbia, Belgrade, Serbia
³Faculty of Medicine University of Belgrade, Department for Anesthesiology, Clinical Center of Serbia, Belgrade, Serbia
4Faculty of Medicine University of Belgrade, Clinic for Physical and Rehabilitation Medicine, Clinical Center of Serbia, Belgrade, Serbia
*Corresponding author: Aleksandar Karamarković, Faculty of Medicine University of Belgrade, Clinic for Emergency Surgery, Clinical Center of Serbia, Pasteur Str.2, Belgrade, Serbia
Received: October 18, 2016; Accepted: December 27, 2016; Published: December 29, 2016
Abstract
Background: We evaluated technique of hepatic resections using suprahilar-extrafascial dissection of Glissonean pedicle with vascular stapling device for pedicle transection with intent to minimize operative time and blood loss.
Methodology: We analyzed the clinical records of 326 patients who underwent anatomic liver resection by suprahilar-extrafascial pedicle isolation with vascular stapling division technique.
Results: The minor liver resections were associated with significantly shorter surgery duration (105.1±21.1 vs. 225.6±75.6) and transection time (40.1±14.5 vs. 96.3±55.2) than major hepatectomies (P<0.0001 for all). The mean blood loss was 350.8±100.5 mL in minor resection and 485.4±250.2 mL in major resection (P=0.001). The mean blood transfusion requirement was 400.8±109.5 mL for minor resections and 550.9±100.0 mL for major hepatectomy (P=0.072). There was no significant difference in morbidity and mortality between groups (P=0.980; P=0.945). Major as well as minor liver resection were an oncology superior with no significant difference in the 5 year overall survival rates.
Conclusions: Extrafascial dissection of Glissonean pedicle represents an effective and safe technique of liver resection. Presented approach allows early and easy ischemic delineation of appropriate liver territory to be removed with selective inflow vascular control. It is not time consuming and it is very useful in re-resection, as well as oncological reasonable.
Keywords: Suprahilar-extrafascial dissection; Vascular stapling; Glissonean pedicle
Introduction
Hepatic resection had an impressive growth, both by broadening the range of its indications and the occurrence of changes and technical tricks in order to reduce postoperative mortality and morbidity [1]. Although the criteria for liver tumors resectability are expanded today, hepatectomies are still demanding procedures due to risk of hemorrhage and hepatic failure [2-6]. During the last decades surgical techniques for hepatectomy have changed dramatically [2-10]. All improvements in liver surgery have the same goals, to preserve the maximum amount of Liver parenchyma with minimum blood loss [1-10]. The blunt liver dissection has been widely replaced by various time-consuming methods, such as the cavitron ultrasonic surgical aspirator (CUSA), followed by the development of tools for safe approach, isolation and transection of vascular and biliary structures during transection of liver parenchyma [8,9]. In 1949, Honjo (Kyoto University) and later in 1952, Lortat-Jacob and Robert were performed the first anatomical right hepatectomy with classical intrafascial-extrahepatic approach so-called “classic” hilar dissection (HD) of the hepatic artery, portal vein and bile duct in the hepatoduodenal ligament [7,8,10]. Nevertheless, the potential disadvantages of this approach are reflected in the cases of extensive scarring due to previous surgery, the risk of incidental lesion of anomalous hepatic vessels or the contralateral biliary duct [11-14]. The observations of Glisson and Couinaud that elements of portal triad are contained within a thick connective tissue and are surrounded by a fibrous sheet (Glissonean pedicle) were the basis for the initial proposal by Couinaud in 1957, that suprahilar vascular control of Glissonean pedicle could serve as an important alternative to classical HD for controlling vascular inflow to the liver. This technique includes the extrafascial dissection of the whole sheath of the pedicle and its division “en masse” [15]. Anterior intrahepatic extrafascial approach proposed by Couinaud, Thung and Quang, uses anatomical fissures as doors of the liver. By splitting the liver substance down along the appropriate fissure could be approach to the pedicle of interest [15,16]. The extrafascial dissection of left Glissonean pedicle at the hepatic hilus without liver transection, for the left hepatectomy, was previously reported by Couinaud in 1985 and later by Lazorthes in 1993 [17,18]. Takasaki in 1986 described the surgical technique called “Glissonean pedicle transection method”. Technique is based on detachment of the hilar plate and extrafascial-extrahepatic dissection of the main left and right, as well as both right sectional pedicles, without opening the liver parenchyma [19,20]. Galperin in 1989 described a digital “hooking” technique for the isolation of portal pedicles through an extrafascial-intrahepatic approach after division of a substantial amount of the hepatic tissue [21]. In 1992 Launois and Jamieson proposed the posterior intrahepatic approach to the appropriate Glissonean pedicle, through the dorsal fissure of the liver, after making proper perihilar hepatotomies [22]. Machado’s modifications of the posterior approach include making small incisions around the hilar plate and strictly instrumental isolation of the pedicle [23-25]. It has been reported that the Glissonean approach (GA) can reduce the portal triad closure time, expedite the transection of the liver and reduce intraoperative hemorrhage, as well as the risk of injury to the vasculature or the biliary drainage of the contralateral liver [26,27]. A step forward in achieving security is the introduction of vascular staplers in liver surgery [8,28-31]. Vascular staplers offer speed and safety when dividing hepatic veins and portal branches during hepatectomy, which minimizes blood loss [8,31]. Previous studies compared classical HD vs. extrahepatic Glissonian stapling of the pedicle for major hepatectomies with acceptable morbidity [7,32]. Using technique of the suprahilar-extrafascial Glissonean pedicle dissection, with endo-GIA vascular stapling device transection of the pedicle, and appropriate hepatic vein, we have performed 326 liver resections for malignant and benign tumors, with intent of minimal blood loss. Here we review our experience gained with liver resections and compare the clinical, perioperative and postoperative results (complications, disease-free survival and overall survival) of the patients who have undergone either segmental resection of different volume, or major hepatectomy.
Methodology
We prospectively analyzed the clinical records of 326 patients who underwent hepatic resection by suprahilar-extrafascial pedicle isolation and stapling technique division in our Clinic for emergency surgery in Belgrade, between January 2008 and December 2015. Patients who underwent hilar extrahepatic intrafascial dissection were excluded from the study. All procedures were performed by the same operating team. The protocol received the approval of the research review board of our hospital, and informed written consent was obtained from each patient before surgery. Before operation, all patients underwent a thorough physical examination, blood tests and radiologic evaluation. Liver function was evaluated by Child-Pugh- Truscott (CPT) classification using prothrombin time (PT), albumin, bilirubin and clinical findings of ascites and encephalopathy. CPT score was stratified as classes A [5-6], B [7-9], and C [10-15]. Only CPT class C is considered an absolute contraindication for surgical treatment. Liver resections were defined according to the International Hepato-Pancreato-Biliary Association terminology derived from Couinaud’s classification [33]. The amount of operative blood lost was measured by the volume (mL) of blood collected in the aspirator container and the ultrasonic dissector and by the weight of the soaked gauzes. Perioperative data were operative duration (min), transection time (min), intraoperative blood loss (mL), transfusion requirement (intraoperative and postoperative within the first 48h) and intermittent vascular occlusion (IVO) duration (min). Transection time was defined as the duration between the beginning and the end of the liver parenchyma transection. The amount of operative blood lost was measured by the volume (mL) of blood collected in the aspirator container and by the weight of the soaked gauzes (assuming that 1mL of blood =1g). The indications for blood transfusion were massive hemorrhage with hematocrit decreasing to approximately <5% or hemoglobin level <70g/L. Cumulative clamping time was calculated according to cumulative period of vascular occlusion. Postoperative data included postoperative liver injury, ICU and hospital stay (days), morbidity and mortality and disease-free survival and overall survival. The patients were subjected to postoperative follow-up by blood test, ultrasonography or computed tomography (CT) scans. The degree of postoperative hepatic injury was assessed by measuring the postoperative serum values of the aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin, PT and international normalized ratio (INR) on postoperative days 1, 3, 5 and 7. Postoperatively were followed in the outpatient clinic at 1, 3, and every 6 months thereafter with blood biochemistry and spiral CT scans of the abdomen. Post-operative mortality was defined as any death occurring within 30 days after surgery. Postoperative bleeding, liver ischemia, bile leakage, or perihepatic abscess formation were considered surgical complications. Biliary leak was defined as any drainage through the catheter with a bilirubin content 2× higher than the plasma levels.
Surgical technique
Makuuchi’s “J”-shaped laparotomy was used for all patients. Liver was mobilized using standard technique. Recently, we preferred anterior approach without initial liver mobilization, especially for the huge right sided tumors (Figure 1). Intraoperative ultrasound (IOUS) was performed to redefine tumor localization in relation to major vascular structures and to determine the transection plane. Extra hepatic “outflow” control was performed after dissection and isolation of major hepatic veins above the liver, whenever it was possible. Ischemic preconditioning (IP) was done to minimize ischemic-reperfusion injury of the liver (IRI). The liver tissue was transected under intermittent hepatic inflow vascular occlusion (IVO) which involves periods of inflow clamping for 15 minutes followed by periods of unclamping for five minutes (mode 15/5). In order to minimize bleeding in minor hepatectomies, selective vascular clamping (SVO) was used as the preferred method of inflow occlusion, particularly in patients with underlying chronic liver disease. Central venous pressure (CPV) was maintained at 0-5mmHg to help reduce back bleeding from hepatic veins. After the transectional line was marked, the liver capsule and liver tissue up to 2cm were divided with harmonic scalpel (“LIGASURE”; Eticon Co, USA). Deeper down transection of the liver substance was performed using the cavitron ultrasonic dissecting aspirator (“CUSA Excel”; Valley lab Inc., Boulder, CO, USA). During dissection, small vessels/ bile ducts were ligated, coagulated or clipped to achieved hemostasis and biliostasis. The major hepatic veins were divided extrahepatically using vascular surgical stapler (Endo GIA Ultra stapler 3.0; Covidien, USA). Suprahilar vascular control of the appropriate Glissonean pedicle was achieved by Machado’s modification of the posterior intrahepatic approach [23,24], or using Takasaki’s technique of extrahepatic dissection and isolation of both primary and secondary branches [19,20] (Figure 2). Clamping the taped Glissonean pedicle, demonstrated the further ischemic demarcation of the appropriate anatomical territory of the interest as well as delineation of the line of transection. During pedicle clamping, the color of the area changes and the tumor location is confirmed by IOUS. Pedicle was divided at the end of the resectional procedure using endo-GIA vascular stapling device (Endo GIA Ultra stapler 3.0; Covidien) (Figure 3). Firm counter traction on the tape was applied during application of the stapler to ensure that the contralateral pedicle was not accidentally ligated. After completed resection, the mono polar irrigated electrocautery was applied to stop minor oozing. The raw surface of the liver was sealed using fibrin glue (Figure 4). Closed suction drainage was used in all patients.
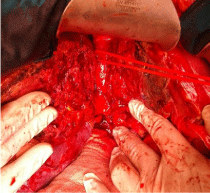
Figure 1: Huge right sided tumor: Anterior transfissural approach without
mobilization of the liver.
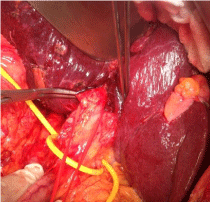
Figure 2: Takasaki’s technique of extrahepatic-extrafascial isolation and
clamping of the right main Glissonean pedicle (RMP) after detachment of
the hilar plate.
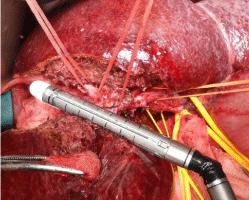
Figure 3: Right inferior hepatectomy: transection of Glissonean pedicle for
the segment S5 using endo-GIA vascular stapling device.
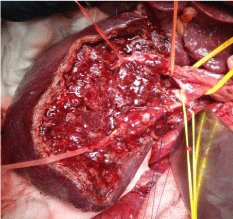
Figure 4: Right sided intrahepatic anatomy of Glissonean tree after removal
segments S5 and S6.
Statistical analysis
Data were expressed as. Differences between groups were compared with parametric Student’s t-test or nonparametric Mann- Whitney test. Repeated measures of liver function indicated by serum level of bilirubin, AST, ALT, albumin and PT were assessed by general linear model. For qualitative variables, comparisons between groups were performed by the χ2 test or Fisher exact test, when needed. In all tests, P value <0.05 was considered to be statistically significant. All the calculations were performed with the SPSS 17.0 statistical package (SPSS, Inc., Chicago, IL, USA).
Results
A total of 326 anatomical hepatectomies were performed by suprahilar-extrafascial Glissonean pedicle dissection and stapling technique, including 127 (39%) major and 199 (61%) minor liver resections (Table 1 and 2). Demographics and preoperative data for all patients are shown in Table 3. There were no significant differences between the two groups in terms of age, gender, comorbid conditions, Child-Pugh score, indications and number of tumoral lesions (Table 3). Forty–nine patients in minor resection group (24.6%) were classified as CPT class B and 37 (29.2%) patients in major resection group as CPT class B. Indications for minor liver resection were metastases of colorectal carcinoma (CRC) in 119 (59.8%), hepatocellular carcinoma (HCC) in 24 (12.1%), cholangiocellular carcinoma in 8 (4.02%), non-colorectal liver metastases in 16 (8.04%), gall bladder carcinoma in 9 (4.52%), hemangioma hepatis in 13 (6.53%) and adenoma hepatis in 10 (5.02%) patients. Indications for major hepatectomies were colorectal liver metastases (CRC LM) in 68 (54.5%); non colorectal liver metastases (non-CRC LM) in 13 (10.23%); HCC in 35 (27.5%); gall bladder carcinoma in 5 (3.93%) patients and liver hemangioma in 6 (4.72%). Intraoperative data for those patients undergoing hepatectomy, hospital stay and outcome are provided in Table 4. There were a significant difference in overall operative time, liver transection time and ischemic duration between minor and major resections (P<0.001 for all) (Table 4). Intraoperative blood loss was significantly higher in the major resection group (P=0.001) (Table 4). Intraoperative transfusion was administrated in 110 (33.7%) patients of all and there was no significant difference between patients with minor and major resections (P=0.395). The intraoperative blood transfusion was expressed as the amount of blood volume (mL), and there was no significant difference between minor and major resections (P=0.072) (Table 4). In 216 (66.3%) patients of all liver resections were performed without intraoperative blood transfusion.
Type of liver resection
n(%)
Segmentectomy 1
4 (2.0%)
Segmentectomy 2
5 (2.5%)
Segmentectomy 3
6 (3.0%)
Left lateral sectionectomy
23 (11.6%)
Left medial sectionectomy (segment 4)
13 (6.5%)
Segmentectomy 5
10 (5.0%)
Segmentectomy 6
14 (7.0%)
Segmentectomy 7
10 (5.0%)
Segmentectomy 8
8 (4.0%)
Right posterior sectionectomy
25 (12.6%)
Right anterior sectionectomy
17 (8.5%)
Bisegmentectomy 4b,5
25 (12.6%)
Bisegmentectomy 3,4b
8 (4.0%)
Right cranial Bisegmentectomy 7,8
10(5.0%)
Right caudal Bisegmentectomy 5,6
21(10.5%)
Total
199 (100.0%)
Table 1: Type of Minor liver resection.
Type of liver resection
n(%)
Extended right hepatectomy
4(3.1%)
Extended left hepatectomy
1(0.8%)
Right hepatectomy
44(34.6%)
Left hepatectomy
47(37.0%)
Mesohepatectomy
9(7.1%)
Central transversal hepatectomy (S3,S4b,S5)
10(7.9%)
Right inferior transversal hepatectomy (S4b,S5,S6)
12(9.5%)
Total
127(100.0%)
Table 2: Type of Major liver resection.
Characteristics
Minor resections (n=199)
Major resections (n=127)
p
Male*
102 (51.3%)
85 (66.9%)
>0.05
Age (years) **
60.13±13.29
62.01±10.23
>0.05
Comorbidity*
109 (54.7%)
81 (63.7%)
>0.05
Malignant indications *
176 (88.4%)
121 (95.2%)
>0.05
No. of tumours lesions**
2,01±1,09
2,13±1,18
>0.05
CPT score A
150(75.4%)
90(70.8%)
>0.05
Bilirubin (µmol/L)
18,12±10,27
21,73±11,56
>0.05
AST (U/L)
30,19±12,21
29,46±12,21
>0.05
ALT (U/L)
39,02±23,40
36,66±29,54
>0.05
Albumin (g/L)
23,19±6,55
21,29±8,81
>0.05
INR†
1,18±0,18
1,44±0,22
>0.05
PT (s)
14,32±1,68
15,22±2,32
>0.05
*Characteristics are presented as numbers of patients and percentage, n (%); **Characteristics are presented as mean ± SD, standard deviation.
Table 3: Clinical characteristics and preoperative biochemical evaluations of patients included in the study.
Characteristics
Minor resections (n=199)
Major resections (n=127)
p
Operative time, (min) **
105.1+21.1
225.6± 75.6
p?0,0001
Transection time, (min)**
40.1±14.5
96.3±65.2
p?0,0001
Blood loss,(ml) **
350.8+100.5
485.4+250.2
p=0,001
Ischaemic duration, min†
15(15)
45(45)
p?0,001
CVP (0-5mm Hg) †
2,00(2)
3,00(2)
0,234
Blood transfusion inraop.(ml)**
400.8+109.5
550.9+100.0
0,072
Resection R0, n (%)*
180(90.4%)
117(92.1%)
0,678
*Characteristics are presented as numbers of patients and percentage, n (%); **Characteristics are presented as mean ±SD. †Characteristics are presented as median (range
Table 4: Perioperative characteristics of patients included in the study.
Degree of liver damage presented by sequential postoperative serum values of AST, ALT, Bilirubin and PT. The changes in postoperative serum values of liver function markers were not significantly different between major and minor resection (P>0.05) Nevertheless, statistical analysis of the total serum AST, ALT, bilirubin, and PT values found significance in the specified period of time. Total AST and ALT values were significantly decreased on the third postoperative day (P<0.001; P<0.001). Total bilirubin value was significantly lower on the 5th postoperative day (P<0.001). Total PT value was significantly reduced on the 5th postoperative day (P=0.001). There was no significant difference in ICU stay, hospital stay and complications rate between the groups (Table 4).
In minor resection group complications rate was 28.6%. According to Clavien’s classification, Grade 1–2 complications were recorded in 45 (22.6%): 5 (2.5%) had cardiac complication, 15 (7.5%) had pleural effusion, 10 (5.0%) had atelectasis, 12 of them (6.0%) had wound infections, 1 (0.5%) had acute gastritis and 2 (1.0%) bronchopneumonia. Total of 12 (6.0%) patients experienced Grade =3 surgery complications: 3 (1.5%) intra-abdominal fluid collection, 2 (1.0%) biliary fistula, 3(1.5%) biloma and 4 (2.0%) partial wound dehiscence. In major resection group according to Clavien’s classification, Grade 1–2 complications were recorded in 34 (26.7%): 4 (3.1%) had cardiac complication, 14 (11.0%) had pleural effusion, 10 (7.9%) had atelectasis and 6 (4.7%) had wound infections. There were 6 (4.7%) Grade =3 surgery complications: 2 (1.6%) intraabdominal fluid collection, 2 (1.6%) biloma and 2 (1.6%) biliary fistula. The majority of complications were treated conservatively, or radiological intervention/percutaneous drainage and no patients underwent reoperation. In all cases of the biliary fistula there was spontaneous healing.
Mortality between groups did not reach a significant difference (P=0.945). The hospital mortality rate in major resections group was 2.9%. All deaths were caused by nonsurgical complications. In two patients there were a history of cardiac disorders, and mortality was caused by an acute myocardial infarction, while third patient ceased due to respiratory insufficiency and severe ventilator associated pneumonia. One patient who treated by minor liver resection died due to thromboembolic complications and pulmonary embolism, on postoperative day 3, despite regular anticoagulant therapy (Table 5).
Characteristics
Minor resections (n=199)
Major resections (n=127)
p
Hospital stay( days) †
6(2)
8(4)
0,845
ICU stay (days)
1,00(2)
1,05(3)
0,541
Morbidity *
57(28.6%)
40(31.5%)
0,880
Mortality rate *
1(0.5%)
3(2.3%)
0,945
Overall survival rates for CRC °
42%
38%
p=0,644
Overall survival rates for HCC °
51%
47%
p=0,788
*Characteristics are presented as numbers of patients and percentage, n (%); †Characteristics are presented as median (range). °Follow-up 60 months.
Table 5: Outcome of liver resections for patients included in the study.
The overall survival rates after 3and 5 years were found to be 53% for patients with colorectal metastases (60% for patients with HCC) and 42% for patients with colorectal metastases (51% for patients with HCC) in group with minor liver resections and 49% for patients with colorectal metastases (69% for patients with HCC) and 38% for patients with colorectal metastases (47% for patients with HCC) in group with major hepatectomies, respectively. There was no significant difference in the overall survival rates between both groups (P=0.788).
Discussion
Liver resections are complex procedures that require detailed knowledge of liver anatomy, precise “bloodless” surgical technique and sufficient volume of the remnant liver [1-8,34]. Since the late 1970s, when operative mortality was more than 20% for major liver resections, much effort has been done to intraoperative control of blood loss and reduce intraoperative hemorrhage [34,35]. Excessive blood loss is associated with increased perioperative morbidity and, in cases of colorectal metastases, a shorter disease-free interval [34,36]. Technical refinements are focused on minimizing hemorrhage during transection of hepatic parenchyma and safe dissection of the major hepatic veins and pedicles [34-36].
The extrafascial dissection of Glissonean pedicle is a very important technique that can be extremely useful in particular circumstances during liver surgery, such as in multi-operated patients or in patients with cirrhotic liver or anomalous vascular and biliary variations. Regarding this technique some terminology confusion still exists (Glissonean approach, extra-Glissonean approach, Glissonean pedicle transection method, posterior intrahepatic approach, suprahilar vascular control, perihilar posterior approach, superficialisation of Glissonean pedicles) [20,37]. Nevertheless, despite many titles the main surgical concept is the same, and it’s based on the anatomical fact and observation of Couinaud that portal triad elements inside the liver substance are enveloped with fibrous Glissonean sheet, thus representing an important structure of internal architecture of the liver [15,17]. The extrafascial-Glissonean pedicle approach in liver surgery provides new knowledge of the surgical anatomy of the liver and advances the technique of liver surgery [38]. Opposite to “classic” intrafascial dissection, this technique includes extrafascial isolation of the whole sheet of Glissonean pedicle and its division “en masse”. Glissonean pedicles can be approached intrahepatically or extrahepatically. The use of vascular staplers in this situation allows quick and safe transection of the pedicle, as well as appropriate hepatic vein [39]. The second advantage of this technique presents the quick and easy definition of the anatomic territory of the liver to be removed. Selective clamping of the appropriate isolated pedicle demonstrates the further ischemic demarcation of anatomical liver part of interest (hemiliver, section or even segment) as well as delineation of resectional planes [21-25]. Recent advances of presented surgical technique includes liver hanging maneuver and some modifications with two tapes to control the main fissure of the liver or various liver resections using hanging maneuver by three Glisson’s pedicles and three hepatic veins [40,41]. The first prospective randomized study which compared extrafascial GA vs. “classic” HD in major hepatectomies, was performed by the group of Figueras, showed that “en bloc” stapling transection of the pedicle was safe and faster than “classic” approach [7]. The other studies have shown similar results for the safety and operative duration [42-46]. Also, the aim of our previous study was to analyze the efficiency and safety of the Glissonean pedicle approach vs. classical HD in major hepatectomies [32]. The extrafascial dissection was associated with significantly shorter surgery duration, transection time and ischemic duration than intrafascial HD, while amount of blood loss was significantly lower in GA [32]. Extrafascial isolation of Glissonean pedicle saves time comparing with difficult and sometime hazardous intrafascial HD. Dissection above hepatic hilum significantly reduces the risk of the potentially injury of the contra-laterally sided vasculature and bile ducts [47]. Smyrniotis et al. showed that intrahepatic dissection is safe as extrahepatic hilar division in terms of intraoperative blood requirements and morbidity; but biliary complications are more severe in patients undergoing extrahepatic division of the portal pedicle [43]. Advantages of anatomic segment orientated resections include prevention of postoperative liver failure especially in elderly or patients with underlying liver disease, reduction of blood loss as well as lower postoperative mortality and morbidity rates. The question, whether to perform a segmental or a major resection if both procedures are technically feasible, is still under debate. The presented surgical technique of suprahilar extrafascial control of the Glissonean pedicle is very useful in performing of sectionectomies and segmentectomies. Couinaud and, more recently, Takasaki, Galperin and Launois have noted that the Glissonean capsule continues within the liver parenchyma up to the segmental divisions [19-22]. Although the inter-segmental planes were not visible on the surface of the liver, the segments were defined by occluding the inflow pedicle to that segment.
This study describes our experiences with the extrafascial pedicle dissection and stapling technique during major liver resection and minor hepatectomy: vascular staplers were used to divided pedicles and major hepatic veins while parenchyma transection was performed by CUSA, under IPM or selective vascular occlusion (SVO). The study was not designed to demonstrate the superiority of one major hepatic resection over the minor. Rather, it is the authors’ intention to demonstrate the efficiency of the GA in major as well as in minor hepatectomy. In our study, bisegmentectomies occupy the greatest relative share in minor liver resection group, since left lateral sectionectomies dominates. In major liver resection group, right hepatectomy and left hepatectomy had the greatest rate. The minor liver resections were associated with significantly shorter surgery duration and transection time than major hepatectomies. Intraoperative transfusion rate was no significant difference between minor and major resections. The changes in postoperative serum values of liver function markers were not significantly different between major and minor resections. There was no significant difference in ICU stay, hospital stay and complications rate between the groups. Major Hepatectomy as well as minor liver resection are a superior oncologic operation with no significant difference in the 3- and 5-year disease-free survival rates and overall survival rates between both groups in our study. Stewart registered a significant difference between the groups with extended resections and segmental ones in terms of operative blood loss and post-operative stay as major post-operative complications are less following segmental resection [48]. Intermittent Pringle maneuver (IPM) during transection of liver parenchyma is simple and safe technique that may reduce bleeding from hepatic inflow, and the total clamping time can be extended to 120 minutes in normal livers and 60 minutes in pathological livers [30,36]. The disadvantage of IPM is that bleeding occurs from the liver transection surface during the unclamping period and, thus, the overall transection time is prolonged as more time is spent in achieving hemostasis. The presented surgical technique allows the use of SVO during parenchymal transection. Selective clamping it is also important from the haemodynamic point of view because there is no splanchnic stasis and low fluid replacement. A previous randomized study demonstrated that the clinical advantages of selective clamping are more significant in patients with chronic liver disease, particularly in very difficult resections in patients in whom lengthy pedicular clamping is anticipated as a result of portal hypertension or in whom very large areas of transection are necessary [49]. By contrast, selective clamping or hemihepatic vascular occlusion, as described by Makuuchi et al. does not increase venous portal pressure or cause fluid overload or a consequent increase in CVP [50].
Expected, in our study results showed shorter operation time, transection time, ischemic duration and less blood loss for minor hepatectomies compared to major liver resections. However our results showed that major hepatic resections are safe procedures with outcome results non-significantly different from minor resections. Further development of sophisticated techniques and instruments in order to reduce bleeding during liver resection led to the introduction of vascular stapler in liver surgery in the last decade of the twentieth century. Recent publications reporting a number of techniques using stapling devices in liver surgery showed them to be extraordinarily useful in the safe ligation of inflow and outflow vessels [51]. Application of vascular staplers to selectively divide major intrahepatic blood vessels for hepatic inflow and outflow vascular control during liver resection, has been shown to achieve excellent results, reducing blood loss, warm ischemia time and operative time [24,26,29]. However, there are a few of potential dangers in using the stapler. Serious blood loss can theoretically occur when the stapler has sealed only half the diameter of the vessel or after misfire of the devise although we did not experience such a situation. Another potential danger from the use of staplers in the liver is tearing a major hepatic vein or vena cava, while placing the instrument. Usually after encircling of the hepatic vein, the articulated and rotating Endo-GIA vascular stapler is passed gently around the hepatic vein to staple and divide it. The thinner blade of the stapler is inserted in preference to the thicker blade because the space available is limited. As the thinner blade is not on the same axis as the instrument, difficulty may be encountered if the tip of the blade and tearing of the vein may occur. In order to avoid this complication, we used a right-angle clamp to grab the thinner blade and guide its insertion into the space between the liver parenchyma and major vein. This technique is also reported by other centers [28]. Morbidity and mortality are correlated with the amount of blood loss during hepatectomy [34,36]. Despite all technological advancing for liver resections, an intraoperative hemorrhage rate ranging from 700 to1, 200mL is reported with a postoperative morbidity rate ranging from 23% to 46% and a surgical death rate ranging from 4% to 5% [34,36]. Jarnagin et al. reported of a moderate blood loss of 600mL and in major hepatectomy their investigations led to a blood loss of more than 1,000mL; while 700 to 800 mL observed in the cases of stapler hepatectomy [35,52]. Specific complications after liver are all associated with high morbidity in terms of sepsis, liver failure, longer hospital stay, as well as postoperative mortality [53,54]. Complications such as biliary leaks continue to be reported with incidences in the range of 2.6-15.6%, in our study1.7% [53,54]. Carefully checking the resection line and completing hemo-and bilistasis, even in a modified cirrhotic liver parenchyma, we obtained literature accepted percentages in resection line related complications (biliary fistulas, postoperative bleeding). Capussotti et al. published a study on 610 patients with liver resection, where biliary fistulas occurred in 3.6% of cases, and our rate of 2.3% of all being consistent with these data [53]. Treatment is not easy and a number of non-surgical strategies have been proposed. However, surgical intervention should be considered for patients in whom non-surgical interventions are either unsuccessful or not feasible. In this study no patients underwent reoperation, all complications treated successfully by non-operative interventional and radiological techniques. In our series, no hemorrhage, ischemic damage or postoperative liver function was observed. Our experience in study of 326 patients who underwent hepatectomy with stapling of the pedicle shows that this technique is applicable in a routine clinical setting based on both its feasibility and safety. Mortality of 1.3% seen in our group is consistent with our previously published results [55], as well as with the data published in the literature. In the present series, both mortality and morbidity were a slow as in a recently published large series of non-selected patients who underwent liver resection in other high volume surgical centers [1,35,52].
Conclusions
Extrafascial dissection of Glissonean pedicle with vascular stapling represents both an effective and safe surgical technique of anatomical liver resection. Presented approach allows early and easy ischemic delineation of appropriate anatomical liver territory to be removed (hemiliver, section, segment) with selective inflow vascular control. Also, it is not time consuming and it is very useful in reresection. From the oncological point of view technique is reasonable: early initial ligation of Glissonean pedicle avoid dissemination of neoplastic cells, while anatomical concept of resection allows removal of micro metastases at the root of the pedicle with adequate resectional margin. We have demonstrated that segment-orientated liver resections offers disease-free and overall survival rates similar to those after major resection. However, the patients should be judiciously selected. Finally, according to our opinion, extrafascial GA should be a part of knowledge and skills of HPB surgeon.
References
- Dan RG, Cretu OM, Mazilu O, et al. Postoperative morbidity and mortality after liver resection. Retrospective study on 133 patients. Chirurgia (Bucur). 2012; 107: 737-741.
- Chirica M, Scatton O, Massault PP, et al. Treatment of stage IVA hepatocellular carcinoma: should we reappraise the role of surgery? Arch Surg. 2008; 143: 538-543.
- Pawlik TM, Schulick RD, Choti MA. Expanding criteria for respectability of colorectal liver metastases. Oncologist. 2008; 13: 51-64.
- González HD, Figueras J. Practical questions in liver metastases of colorectal cancer: general principles of treatment. HPB (Oxford). 2007; 9: 251-258.
- Minagawa M, Yamamoto J, Miwa S, et al. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006; 141: 1006-1012; discussion 1013.
- Nuzzo G, Giuliante F, Ardito F, et al. Liver resection for primarily unresectable colorectal metastases downsized by chemotherapy. J GastrointestSurg. 2007; 11: 318-324.
- Figueras J, Lopez-Ben S, Lladó L, et al. Hilar dissection versus the ‘Glissonian’ approach and stapling of the pedicle for major hepatectomies: a prospective, randomized trial. AnnSurg. 2003; 238: 111-119.
- Aragon RJ, Solomon NL. Techniques of hepatic resection. J GastrointestOncol. 2012; 3: 28-40.
- Doklestic K, Karamarkovic A, Stefanovic B, et al. The efficacy of three transection techniques of the liver resection: a randomized clinical trial. Hepatogastroenterology. 2012; 59: 1501-1506.
- Lortat-Jacob JL, Robert HG. HepatectomiedroitereglÉ. Press Med. 1952; 60: 549-551.
- Meunier B, Lakehal M, Tay KH, et al. Surgical complications and treatment during resection for malignancy of the high bile duct. World J Surg. 2001; 25: 1284-1288.
- Lo CM, Fan ST, Liu CL, et al. Biliary complications after hepatic resection: risk factors, management and outcome. Arch Surg. 1998; 133: 156-161.
- Miyagawa S, Makuuchi M, Kawasaki S, et al. Criteria for safe hepatic resection. Am J Surg. 1995; 169: 589-594.
- Icoz G, Kilic M, Zeytunlu M, et al. Biliary reconstructions and complications encountered in 50 consecutive right lobe living donor liver transplantation. Liver Transpl. 2003; 9: 575-580.
- Couinaud C. Le foie. Études Anatomiques et Chirugicales. Paris: Masson. 1957.
- Tung TT. Les RÉsections Majeures et Mineures du Fois. Paris: Masson &Cie. 1957.
- Couinaud CM. A simplified method for controlled left hepatectomy. Surgery. 1985; 97: 358-361.
- Lazorthes F, Chiotasso P, Chevreau P, et al. Hepatectomy with initial suprahilar control of intrahepatic portal pedicles. Surgery. 1993; 113: 103-108.
- Takasaki K. eds. Glissonean pedicle transection method for hepatic resection. Tokyo: Springer. 2007.
- Takasaki K. Glissonean pedicle transection method forhepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg. 1998; 5: 286-291.
- Galperin EI, Karagiulian SR. A new simplified method of selective exposure of hepatic pedicles for controlled hepatectomies. HPB Surgery. 1989; 1: 119-130.
- Launois B, Jamieson GG. The importance of Glisson’s capsule and its sheaths in the intrahepatic approach to resection of the liver. Surg Gynecol Obstet. 1992; 174: 7-10.
- Machado MA, Herman P, Machado MC. A standardized technique for right segmental liver resections. Arch Surg. 2003; 138: 918-920.
- Machado MA, Herman P, Machado MC. Anatomical resection of left liver segments. Arch Surg. 2004; 139: 1346-1349.
- Machado MA, Herman P, Meirelles RF Jr, et al. How Ido it: bi-segmentectomy V-VIII as alternative to right hepatectomy: an intrahepatic approach. J Surg Oncol. 2005; 90: 43-45.
- Fong Y, Blumgart L. Useful stapling techniques in liver surgery. J Am Coll Surg. 1997; 185: 93-100.
- Ramacciato G, Aurello P, D’Angelo F, et al. Effective vascular endostapler techniques in hepatic resection. Int Surg. 1998; 83: 317-323.
- Wang WX, Fan ST. Use of the Endo-GIA vascular stapler for hepatic resection. Asian J Surg. 2003; 26: 193-196.
- DeMatteo RP, Fong Y, Jarnagin WR, et al. Recent advances in hepatic resection. Semin Surg Oncol. 2000; 19: 200-207.
- Schemmer P, Friess H, Hinz U, et al. Stapler hepatectomy is a safe dissection technique: analysis of 300 patients. World J Surg. 2006; 30: 419-430.
- Bagul A, McMahon G, Alam F, et al. Safely dealing with the right hepatic vein during a right hepectomy. Ann R Coll Surg Engl. 2010; 92: 442-443.
- Karamarković A, Doklestić K, Milić N, et al. Glissonean pedicle approach in major liver resections. Hepatogastroenterology. 2012; 59: 1896-1901.
- Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford). 2002; 4: 99; 99-100.
- Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004; 240: 698-708; 708-710.
- Poon RT. Current techniques of liver transection. HPB (Oxford). 2007; 9: 166-173.
- Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred fourty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000; 191: 38-46.
- Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982; 6: 3-9.
- Yamamoto M, Katagiri S, Ariizumi S, et al. Glissoneanpedicle transection method for liver surgery. J Hepatobiliary Pancreat Sci. 2012; 19: 3-8.
- Nanashima A, Sumida Y, Oikawa M, et al. Vascular transection using endovascular stapling in hepatic resection. Hepatogastroenterology. 2009; 56: 498-500.
- Boudjema K, Veilhan LA, Dupont-Bierre E, et al. Two tapes to control the main fissure of the liver. Ann Chir. 2002; 127: 149-153.
- Kim SH, Park SJ, Lee SA, et al. Various liver resections using hanging maneuver by three Glisson’s pedicles and three hepatic veins. Ann Surg. 2007; 245: 201-205.
- Cresswell AB, Welsh FK, John TG, et al. Evaluation of intrahepatic, extra-Glissonian stapling of the right porta hepatis vs. classical extrahepatic dissection during right hepatectomy. HPB (Oxford). 2009; 11: 493-498.
- Smyrniotis V, Arkadopoulos N, Theodoraki K, et al. Association between biliary complications and technique of hilar division (extrahepatic vs. intrahepatic) in majorliver resections. World J Surg Oncol. 2006; 4: 59.
- Giordano M, Lopez-Ben S, Codina-Barreras A, et al. Extra-Glissonian approach in liver resection. HPB (Oxford). 2010; 12: 94-100.
- Mouly C, Fuks D, Browet F, et al. Feasibility of the Glissonian approach during right hepatectomy. HPB (Oxford). 2013; 15: 638-645.
- Yamamoto M, Katagiri S, Ariizumi SI, et al. Tips for anatomical hepatectomy for hepatocellular carcinoma by the Glissonean pedicle approach. J Hepatobiliary Pancreat Sci. 2014; 21: E53-56.
- Launois B. General principles of liver surgery. In: Launois B, Jamieson G. eds. The posterior intrahepatic approach in Liver surgery. New York, USA: Springer. 2013.
- Stewart GD, O’Súilleabháin CB, Madhavan KK, et al. The extent of resection influences outcome following hepatectomy for colorectal liver metastases. Eur J Surg Oncol. 2004; 30: 370-376.
- Figueras J, Llado L, Ruiz D, et al. Complete versus selective portal triad clamping for minor liver resections. A prospective randomized trial. Ann Surg. 2005; 241: 582-590.
- Makuuchi M, Mori T, GunvÉn P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987; 164: 155-158.
- Delis SG, Bakoyiannis A, Karakaxas D, et al. Hepatic parenchyma resection using stapling devices: peri-operative and long-term outcome. HPB (Oxford). 2009; 11: 38-44.
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection. Analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002; 236: 397-406; 406-407.
- Capussotti L, Ferrero A, Vigano L, et al. Bile leakage and liver resection: where is the risk? Arch Surg. 2006; 141: 690- 694; 695.
- Nagano Y, Togo S, Tanaka K, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003; 27: 695-698.
- Karamarković A, Doklestić K, Popović N, Gregorić P, Vujadinović ST, Milić N. Suprahilar vascular control and stapling device transection of Glissonian pedicle in major and minor hepatectomies. Hepato-Gastroenterol. 2013; 60: 2060-2068.