Review Article
Ann Surg Perioper Care. 2017; 2(1): 1022.
The Role of Breast Ultrasound in a Treatment Protocol for the Postoperative Red Breast in Implant Based Breast Reconstruction
Grome L¹*, Sears S¹, Warner R¹ and Mason A²
¹School of Medicine, West Virginia University, West Virginia, USA
²Department of Surgery, West Virginia University, West Virginia, USA
*Corresponding author: Grome L, School of Medicine, West Virginia University, PO Box 9238, Health Sciences Center, One Medical Center Drive, Morgantown, WV 26506-9238, West Virginia, USA
Received: February 10, 2017; Accepted: March 06, 2017; Published: March 13, 2017
Abstract
Patients presenting with a red breast after implant based breast reconstruction represent a diagnostic challenge. This paper reviews the six most common causes of red breast including infection, seroma, hematoma, radiation, tissue necrosis and red breast syndrome. Pathologic presentation and treatment modalities are also discussed. Additionally we suggest an algorithm for the management of red breast that utilizes ultrasound as a differentiating tool to help solve the diagnostic problem of when conservative management, antimicrobial therapy and or explantation are appropriate.
Keywords: Red Breast; Tissue Expander; Breast Reconstruction; Ultrasound
Introduction
Patients undergoing implant based breast reconstruction that return post operatively with a red breast can present a diagnostic challenge. Six of the most common causes of red breast include seroma, hematoma, infection, radiation, vascular insufficiency, and acellular dermal matrix hypersensitivity (red breast syndrome). A focused approach to the evaluation of a red breast is vital in ruling out pathology that warrants expander explantation. Understanding of the entities that may lead to a red breast in the postoperative period and a protocol for evaluation and treatment is essential in differentiating those patients who need emergent explantation, trailed for salvage, or have no infectious processes.
Postoperative Infection
Infection is the most common postoperative cause of erythema following breast reconstruction utilizing tissue expanders. Morbidity resulting from infection is significant and includes lengthy hospitalizations, readmission, delay of chemotherapy and explanation. The rate of infection following reconstructive surgery after mastectomy for breast cancer is an estimated 5.7 percent for first stage tissue expander patients, 2.5 percent for second-stage, and 9.9 percent for direct-to-implant reconstruction. Forty-seven to 71 percent of surgical-site infection complications were late surgical-site infections occurring between 31 days and 1 year [1]. Risk factors for infection include a BMI greater than 30 [2] the use of aseptic ADM [3], diabetes, seroma formation, and mastectomy skin flap necrosis [4]. The average time to infection is 30.7 days post operatively [4].
Patients present with an erythematous, painful breast, with or without associated fever. Induration and a poorly defined but expanding margin of erythema may be present in cases of cellulitis. Serum markers of inflammation including white count, erythrocyte sedimentation rate, and C-reactive protein levels are commonly elevated [4]. Staphylococcusepidermidis is the most common cause of postoperative infection after reconstructive breast surgery, followed by Group B strep, and Morganella morganii [4]. Staph epidermidis’ ability to form a protective biofilm on the surface of prostheses makes bacterial clearance more difficult, often leading to periprosthetic collections and device explantation. Other organisms implicated in these infections include Methicillin sensitive Staphylococcus aureus (MSSA), Serratiamarcescens, Pseudomonasaeruguinosa, enterococcus species, Escherichia coli, Enterobacter species and Methicillin resistant Staphylococcus aureus (MRSA) [4]. Treatment focuses on antimicrobial therapy in an attempt to prevent tissue necrosis, abscess formation, sepsis, and explanation. Oral broadspectrum antibiotics and careful monitoring for response are often used first line for superficial infections proximal to the incision line [1]. If symptoms do not improve with oral therapy or if diffuse cellulitic changes are present, intravenous antibiotics should be initiated and the device may need explanted [4]. Oral antibiotics used should exhibit high sensitivity and low resistance profiles. Studies demonstrate that fluoroquinolones should be used as first line oral therapy; these include Levofloxacin (sensitivity 80%, resistance 13%) and Ciprofloxacin (sensitivity 63%, resistance 20%) [4]. Intravenous therapies shown to be effective include Vancomycin (sensitivity 60%, resistance 0%), Gentamicin (sensitivity 86%, resistance 0%) and Imipenem (sensitivity 63% resistance 10%) [4]. Failure of intravenous therapies necessitates explanation [5].
Seroma/Hematoma
Patients undergoing breast reconstruction using tissue expanders and acellular dermal matrix (ADM) have a demonstrated increased rate of post-operative fluid collections including seroma and hematoma [6,7]. Most seromas are transient with spontaneous resolution [8]. Their incidence varies widely from 15% to 90% [9]. The incidence of hematomas after breast surgery is reported to be 1-6% [10]. Though the etiologies and components of the collections differ, both hematomas and seromas may present as a red breast in the postoperative patient. Clinically, patients note an enlargement of the breast, along with erythema and tenderness. Depending on the size of the collection, the overlying tissue may become firm [11]. The timing of presentation may vary but most hematomas present within the first week after surgery [12]. Breast ultrasonography is the preferred imaging modality to distinguish between hematoma and seroma. Prevention of seroma and hematoma are paramount in the execution of sound surgical technique. A dry operative field decreases the risk of hematoma formation. Strategies to reduce seroma development include the use of drains. Placing drains at the end of surgery has been shown to decrease the rate of seroma formation from 16 to 5 percent [11]. Shoulder immobility has not been shown to reduce the incidence of seromas [9]. Clinically significant seromas can be managed by needle aspiration, while symptomatic hematomas require open drainage [11].
Radiation
Adjuvant radiation therapy in conjunction with mastectomy and chemotherapy is often a component of breast cancer treatment regiments. Radiation therapy employs ionizing radiation to irreversibly damage the DNA of malignant cells. While radiation is targeted to the breast neoplasm, collateral cellular damage of non-neoplastic tissue occurs. Detrimental effects on healthy tissue result in acute, subacute and chronic changes, with over 80% of patients experiencing a moderate to severe reaction of the overlying skin [13]. The number of treatments, the type of radiation used, and the nutritional status of the patient can impact radiation effects on the skin over the area treated [14]. This effect is known as known as radiation dermatitis. For an interval of three weeks post treatment, erythema and pigment changes may occur secondary to augmented regional blood flow as well as the release of degradative enzymes [14]. Acute ulceration and or epidermal necrosis may also occur. Dry desquamation occurs at three to six weeks, followed by moist desquamation [14]. Maddock- Jennings found that 87 percent of patients experience moderate to severe radiation dermatitis. An awareness of a patient’s radiation treatment course assists in differentiating erythema of the breast as radiation injury. Meticulous skin hygiene, moisturization and topical applications of hydrocortisone and aloe vera gels are used to treat radiation dermatitis [14].
Flap Necrosis
Necrosis of skin flaps can lead to substantial morbidity and may present as a red breast. Infection, implant exposure, and ultimately implant loss are consequences of flap necrosis [15]. Flap necrosis is noted to complicate up to 7% of implant-based reconstructions [15]. Ischemia of the skin overlying a breast implant leads to a breakdown of the barrier between the implant and the external environment. Breakdown of this skin barrier increases the risk of infection and extrusion. Patient risk factors for skin ischemia include smoking, large breast size, and obesity [16]. Controlling for modifiable patient risk factors such as smoking, in addition to assessment of flap viability intraoperatively, can prevent ischemia related flap necrosis. Affected patients present with overlying skin color changes ranging from pale secondary to insufficient vascular supply to purple and congested. Erythema and blisters may appear on the flap as the skin begins to necrose. Finally a black eschar develops. Early recognition and timely treatment is paramount in preventing expander loss. Salvage of the implant after diagnosis can be achieved with early excision of nonviable skin and re-closure [16].
Hypersensitivity to Acellular Dermal Matrix (Red Breast Syndrome)
Hypersensitivity to Acellular Dermal Matrix or Red Breast Syndrome (RBS) is described as a non-infectious, erythematous breast, occurring in women who have had breast reconstruction with acellular dermal matrix (ADM) [17]. Unlike an infectious etiology, the presentation of RBS lacks fever, pain, warmth and other signs of acute inflammation [17]. Further the area of erythema is limited to the area over the site of the acellular dermal matrix [18]. The incidence of RBS is not known due to its relatively recent recognition and difficulty distinguishing the syndrome from infectious causes of erythema [19]. Prevalence estimates range from 3 to 10% of women who present with breast erythema post ADM implantation [17,18,20]. It has been postulated that a hypersensitivity reaction of the overlying tissue to chemicals used in ADM production may result in regional erythema [17]. Inflammatory markers including C-reactive protein and sedimentation rate, though typically not elevated to the degree as those seen in infectious processes, may be elevated. Pharmacologic treatment options include corticosteroids and montelukast [21]. With or without intervention, red breast syndrome is self-limited and resolves spontaneously over the course of weeks to months [21].
Discussion
Taking the above into consideration, a strategic protocol in the initial workup and treatment of the patient presenting with a red breast during the postoperative period is useful. The primary goal being to exclude flap necrosis or an infectious process that would lead to device loss. An initial complete history and physical examination will typically identify flap necrosis and radiation dermatitis. The suspicion of an infectious process requires a consideration for explantation versus an attempt at treatment and salvage. Below is an algorithm (Figure 1) developed to guide decision making during this process. Workup includes a data set of vital signs, markers of inflammation including leukocyte count, C reactive protein, and a breast ultrasound. The breast ultrasound assists in excluding hematoma and a periprostheticseroma collection. The latter is
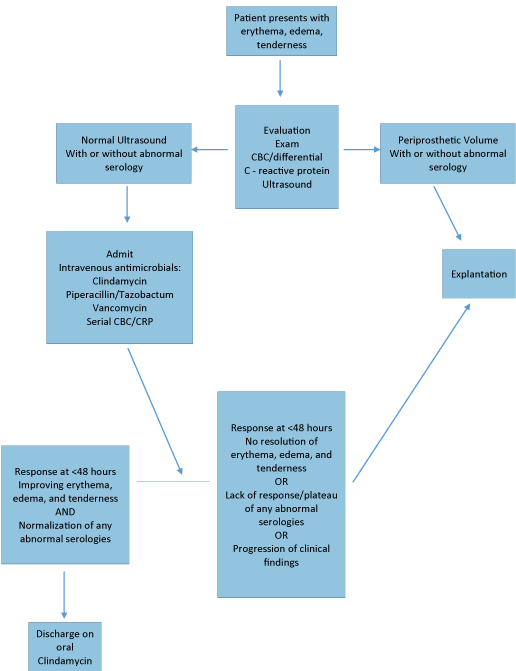
Figure 1: Treatment Protocol for the Postoperative Red Breast in Implant Based Breast Reconstruction.
References
- Sinha I, Pusic AL, Wilkins EG, Hamill JB, Chen X, Kim HM, et al. Late Surgical-Site Infection in Immediate Implant-Based Breast Reconstruction. Plast Reconstr Surg. 2017; 139: 20-28.
- Yuen J, Yue C, Erickson S, Cooper S, Boneti C, Henry-Tillman R, et al. Comparison between freeze-dried and ready- to-use AlloDerm in alloplastic breast reconstruction. Plast Reconstr Surg Glob Open. 2014; 2: e119.
- Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013; 132: 725–736.
- Weichman KE, Levine SM, Wilson SC, Choi M, Karp NS. Antibiotic Selection for the Treatment of Infectious Complications of Implant-Based Breast Reconstruction. Annals of Plastic Surgery. 2013; 71: 140-143.
- Wu P, Winocour S, Jacobson S. Red Breast Syndrome: A Review of the Available Literature. Aesth Plast Surg. 2015; 39: 227–230.
- Michelotti BF, Brooke S, Mesa J, Wilson MZ, Moyer K, Mackay DR, et al. Analysis of Clinically Significant Seroma Formation in Breast Reconstruction Using Acellular Dermal Grafts. Ann Plast Surg. 2013; 71: 274-277.
- Zhao X, Wu X, Dong J, Liu Y, Zheng L, Zhang L. A Meta-analysis of Postoperative Complications of Tissue Expander/Implant Breast Reconstruction Using Acellular Dermal Matrix. Aesthetic Plast Surg. 2015; 39: 892-901.
- Sabel S. Essentials of Breast Surgery: A Volume in the Surgical Foundations Series. Elsevier Health Sciences. 2009; 177.
- vanBemmel AJ, van de Velde CJ, Schmitz RF, Liefers GJ. Prevention of seroma formation after axillary dissection in breast cancer: a systematic review. Eur J Surg Oncol. 2011; 37: 829-835.
- Collins JB, Verheyden CN. Incidence of breast hematoma after placement of breast prosthesis. Plast Reconstr Surg. 2012; 129: 413e-420e.
- Shestak K. Reoperative Plastic Surgery of the Breast. 1st Edition. LWW; 2005.
- Ganske I, Verma K, Rosen H, Eriksson E, Chun YS. Minimizing complications with the use of acellular dermal matrix for immediate implant-based breast reconstruction. Ann Plast Surg. 2013; 71: 464-470.
- Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2000; 48: 1307–1310.
- Maddocks-Jennings W, Wilkinson J, Shillington D. Novel approaches to radiotherapy-induced skin reactions: a literature review. Complementary therapies in Clinical Practice. 2005; 11: 224-231.
- Kanuri A, Liu AS, Guo L. Whom should we SPY? A cost analysis of laserassisted indocyanine green angiography in prevention of mastectomy skin flap necrosis during prosthesis-based breast reconstruction. Plast Reconst surg. 2014; 133: 448e-454e.
- Antony AK, Mehrara BM, McCarthy CM, et al. Salvage of tissue expander in the setting of mastectomy flap necrosis: A 13-year experience using timed excision with continued expansion. Plast Reconstr Surg. 2009; 124: 356–363.
- Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009; 124: 1743– 1753.
- Wu C, Cipriano J, Osgood G Jr, Tepper D, Siddiqui A. Human acellular dermal matrix (AlloDerm®) dimensional changes and stretching in tissue expander/implant breast reconstruction. J Plast Reconstr Aesthet Surg. 2013; 66: 1376–1381.
- Wu P, Winocour S, Jacobson S. Red Breast Syndrome: A Review of the Available Literature. Aesth Plast Surg. 2015; 39: 227–230.
- Hill JL, Wong L, Kemper P, Buseman J, Davenport DL, Vasconez HC. Infectious complications associated with the use of acellular dermal matrix in implant-based bilateral breast reconstruction. Ann Plast Surg. 2012; 68: 432–434.
- Ganske I, Hoyler M, Fox SE, Morris DJ, Lin SJ, Slavin SA. Delayed hypersensitivity reaction to acellular dermal matrix in breast reconstruction: the red breast syndrome? Ann Plast Surg. 2014; 73: S139–S143.