Special Article – Bariatric Surgery
Ann Surg Perioper Care. 2017; 2(2): 1026.
Gastric Leak by Staple-Line Rupture after Sleeve Gastrectomy for Morbid Obesity
Frattini F*, Lavazza M, Delpini R, Rausei S and Dionigi G
Research Center for Endocrine Surgery, University of Insubria, Varese, Italy
*Corresponding author: Francesco Frattini, Research Centre for Endocrine Surgery, University of Insubria, Varese, Italy
Received: April 05, 2017; Accepted: April 28, 2017; Published: May 05, 2017
Introduction
Gastric leak is the most fearing complication following sleeve gastrectomy. It occurs in 1-3% of cases and involves the proximal third of the gastric tube just below the esophago-gastric junction (EGJ) in most cases [1].
We report a case of gastric leak after sleeve gastrectomy in which endoscopy resulted useful in assessment of the vascularization of the tissue close to the gastric leak.
Case Presentation
A 42-year-old, BMI 42kg/m² female, suffering from arterious hypertension underwent laparoscopic sleeve gastrectomy. Intraoperative indocyanine-green enhanced fluorescence imaging showed proper vascularization of the gastric tube (Figure 1). A barium swallow on second postoperative day showed no leak or stenosis (Figure 2). A gastric fistula at the EGJ appeared 5 days after surgery. The patient presented fever, abdominal pain, dispnea, leukocytosis and high values of CRP. A thoraco-abdominal computed tomography (CT) scan with oral contrast detected a gastric leak just below the EGJ supplying a left sub diaphragmatic collection extended all over the spleen (Figure 3 and 4).
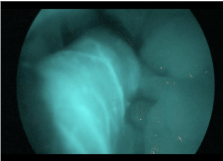
Figure 1:
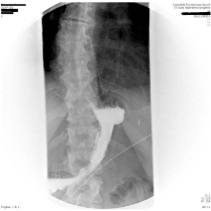
Figure 2:
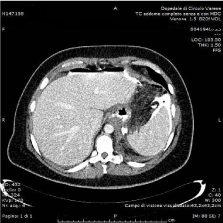
Figure 3:
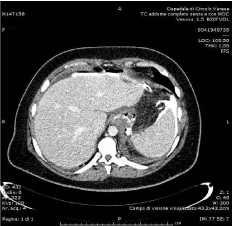
Figure 4:
The patient, then, underwent laparoscopy, cleaning of the left sub phrenic abscess and drainage. No suture of the leak was attempted because of the severe local tissue inflammation. The gastric leak was partially buffered by omentum and flogistic tissue. A left pleuric effusion was drained.
Total parenteral nutrition, endovenous therapy with broadspectrum antibiotic and somatostatine analogue was initiated. Clinical condition improved. White blood cell and CRP progressively decreased.
A CT scan performed 4 weeks after reoperation showed the resolution of the left sub phrenic collection and the persistence of a pseudodiverticular 2cm gastric leak without free passage of contrast from gastric lumen to perigastric/peritoneal cavity and/or to the external drain (Figure 5). In order to plan endoscopic placement of a self-expandable covered stent, an upper gastrointestinal endoscopy was performed. It demonstrated the fistula orifice 4 cm below the cardias with two edges of a staple-line rupture with granulation tissue and well-vascularized gastric mucosa. Injection of contrast medium showed a small pseudodiverticular collection without free extravasation. No esophageal stent was deployed. One week after, antibiotic therapy and somatostatin analogue were interrupted and feeding was restarted. The patient’s recovery was uneventful and the patient was discharged.
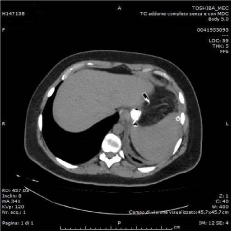
Figure 5:
Discussion
Current classification of gastric leak after sleeve gastrectomy considers time of onset, clinical presentation and size of the leak [2,3].
Gastric leak causes include staple misfiring, wrong choice of the stapler cartridge related to the gastric wall thickness, stenosis of the gastric tube and devascularization of EGJ following sleeve gastrectomy [4,5]. Smoking, steroidal therapy, diabetes may be considered contributing factors.
Gastric leak pathogenesis is still a controversial and debating issue. Impaired vascular supply of EGJ is hardly defined in an objective way and quantifiable.
Nonetheless ischemia of the EGJ is strictly related to the severity of the gastric leak, a more demanding treatment, more difficult healing and prolonged healing time. Evaluation of the vascular supply of the proximal third of the gastric sleeve tissue could be of utmost importance in order to provide the most appropriate treatment option.
In the reported case, endoscopy was useful to detect tissue perfusion surrounding the gastric leak and to clarify the pathogenesis of the leak itself. As evidenced by the endoscopic evaluation, the gastric leak was probably due to a staple-line rupture without any ischemic damage. As a consequence of this evidence it was decided to restart feeding without any further endoscopic procedure.
Conclusion
In conclusion, we believe that it is very important not only to consider time of onset and clinical-pathological features of a gastric leak following sleeve gastrectomy but also to detect its own pathogenesis and the vascular supply of the gastric tube. These items could condition management of the gastric leak and predict the outcome.
References
- Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014; 10: 713-723.
- Csendes A, Diaz JC, Burdiles P, et al. Classification and treatment of anastomotic leakage after extended total gastrectomy in gastric carcinoma. Hepatogastroenterology. 1990; 37: 174–177.
- Nedelcu M, Manos T, Cotirlet A, Noel P, Gagner M. Outcomes of leaks after sleeve gastrectomy based on a new algorithm addressing leak size and gastric stenosis. Obes Surg. 2015; 25: 559-563.
- Iossa A, Abdelgawad M, Watkins BM, Silecchia G. Leaks after laparoscopic sleeve gastrectomy: overview of pathogenesis and risk factors. Langenbecks Arch Surg. 2016; 401: 757-766.
- Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M. The science of stapling and leaks. Obes Surg. 2004; 14: 1290-1298.