Case Report
Ann Surg Perioper Care. 2017; 2(2): 1027.
Surgical Treatment of Left Atrioventricular Fistula due to Mitral Annular Abscess after Mitral Valve Replacement for Active Endocarditis
Nakanishi K*, Tambara K and Saito Y
Department of Cardiovascular Surgery, Juntendo University, Shizuoka Hospital, Shizuoka, Japan
*Corresponding author: Keisuke Nakanishi, Department of Cardiovascular Surgery, Juntendo University Shizuoka Hospital, 1129 Nagaoka, Izunokunishi, Shizuoka, Japan
Received: May 04, 2017; Accepted: May 26, 2017; Published: June 02, 2017
Abstract
Mitral valve annular abscess with fistula is a life-threatening complication of mitral valve endocarditis. Surgical treatment is yet to be established, and operative mortality rate remains high. In the present case, severe mitral regurgitation due to left ventricular atrium fistula was diagnosed using echocardiography 3 months after the patient underwent mitral valve replacement for active mitral valve endocarditis. The fistula was found at the posterior site of the mitral valve annulus. Severe destruction of the annulus had caused the prosthetic valve to become unstable. Surgical procedures involved resection of weakened tissue of the annulus, fistula closure with a bovine pericardial patch using 5-0 Prolene suture, and mitral valve re-replacement. The postoperative course was good, without any complication. This case report demonstrates that patch closure using a bovine patch and re-replacement of the mitral valve was feasible for the rare mitral valve annulus complication.
Keywords: Mitral annular abscess; Endocarditis; Left atrioventricular fistula; Cardiac surgery
Introduction
Mitral annular abscess is a rare complication of infective endocarditis, which could lead to life-threatening complications such as annular rupture or fistula formation. Extension of infective endocarditis beyond the valve annulus is associated with a higher mortality rate, cardiac heart failure, and the need for surgical intervention [1]. Annular extension of infective endocarditis causes annular enlargement pseudoaneurysm, and atrioventricular block [2]. Herein, we report a successful surgical treatment for peri-annular fistula due to infective endocarditis.
Case Presentation
A 46-year-old male underwent mitral valve replacement for endocarditis of the mitral valve. During the operation, mitral valve destruction was found to be severe; thus, valve replacement was decided. In the first operation, left ventricular perforation occurred from below the annulus to the intra-atrial wall, which was covered by a pericardial patch. Three months after the operation, the patient was febrile, hada blood pressure of 80/60mmHg, and hada low-volume pulse at a rate of 130/min. Chest examination disclosed bilateral diffuse fine crackles. Cardiovascular examination revealed a loud and harsh grade 5/6 pan systolic murmur at the apex, with radiation to the axilla, and a third heart sound. Electrocardiography revealed sinus tachycardia with features indicative of left atrial overload. Chest radiography (posterior anterior view) showed bilateral diffuse edema of the lung, and pleural effusion was suspected. Two-dimensional transthoracic echocardiography (apical four-chamber view) revealed a severe mitral regurgitation jet coming from the perforation and an echo-free cavity at the medial mitral annulus, with a 3mm perforated opening into the left atrium (Figure 1). No evidence of vegetation, thrombus, or other significant structural abnormalities was found. Blood culture from the patient showed no growth, although the past culture had revealed Staphylococcus epidermidis.
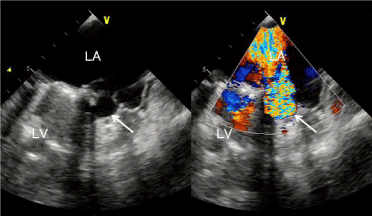
Figure 1: Transesophageal echocardiogram showing the free cavity space at
the mitral valve annulus (solid arrow). The shunt flow jet from the cavity to the
left atrium comprises a mosaic of various colors.
The patient was treated with diuretics, parenteral antibiotics, and routine intensive care, while urgent surgical intervention was immediately decided. Surgical repair was performed through a median sternotomy. Cardiopulmonary bypass was established after heparinization, and the left atrium was opened under hypothermic cardiac arrest. The annular fistula revealed the posterior site of a mitral valve annulus sized 15 x 10 mm. The annulus destruction was severe, which made the prosthetic valve unstable. The weakened tissue was resected, and the fistula was closed with a bovine pericardium patch using 5-0 Prolene suture. Mitral valve re-replacement was then performed (SJM 29mm). The anoxic arrest time was 131min at 35.4°C. After rewarming, the patient was easily weaned from cardiopulmonary bypass without arrhythmia, and his postoperative hemodynamic state was stable. No capillary hydrodynamic fractionation or mechanical support was required. A low-dose catecholamine infusion was administered, and the patient was extubated on postoperative day 1. He was transferred to the general ward on postoperative day 2 and discharged without complications on postoperative day 16. Postoperative echocardiography revealed no mitral regurgitation and no fistula on the mitral valve annulus.
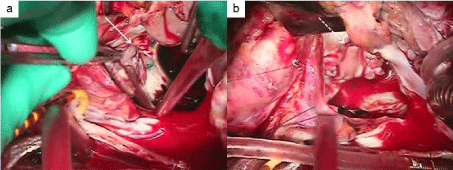
Figure 2: The fistula at the posterior side of the mitral annulus (a, solid arrow).
The fistula was closed with a bovine patch by using 5-0 Prolene suture (b,
dotted arrow).
Discussion
Mitral valve annular abscess with fistulais a life threatening complication of mitral valve endocarditis. Surgical treatment is yet to be established, and operative mortality rate remains high. Fistula of left-sided endocarditis occurs in 5% of the cases [2]. Fistulas and pseudoaneurysms are the result of peri-annular extension of endocarditis with tissue necrosis and liquefaction, leading to communication between, or external to, the cardiac chambers. The incidence of fistulas/pseudoaneurysms is much higher for annular abscess of the mitral valve than for that of the aortic valve [3]. According to a study conducted by Fritz et al. [3], operative mortality and major morbidity were higher in surgical endocarditis cases with annular abscess than in those without, although only the latter was statistically significant. Although intuitively likely, various studies differ as to whether annular abscess is indeed a significant risk factor of operative mortality [3]. Autologous pericardial patches for annular repair and mitral valve replacement for destructed valve annulus were recommended. For annular reconstruction, either an autologous pericardial or bovine pericardium patch was recommended [4]. Surgical treatment for patients with perivalvular extension usually involves valve replacement and a customized approach toward the abscess, depending on its extent, including drainage, necrotic tissue removal, and closure of accompanying fistulas. However, the presence of perivalvular abscess and preoperative shock has been associated with elevated operative mortality. Long-term survival is adversely affected by age and recurrent infective endocarditis [5].
Conclusion
Perivalvular fistula after mitral valve replacement is rare. This case report demonstrates that patch closure of a perivalvular fistula and rereplacement of the mitral valve was feasible to treat left ventricular atrial fistula after mitral valve replacement for active endocarditis.
References
- Yamaguchi H, Eishi K. Surgical treatment of active infective mitral valve endocarditis. Ann Thorac Cardiovasc Surg. 2007; 13: 150-155.
- Graupner C, Vilacosta I, San Román JA, Ronderos R, Sarriá C, Fernández C, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol. 2002; 39: 1204-1211.
- Baumgartner FJ, Omari BO, Robertson JM, Nelson RJ, Pandya A, Pandya A, et al. Annular abscesses in surgical endocarditis: anatomic, clinical, and operative features. Ann Thorac Surg. 2000; 70: 442-427.
- Ishikawa S, Kawasaki A, Neya K, Abe K, Suzuki H, Koizumi S, et al. Surgical treatments for infective endocarditis involving valve annulus. Ann Thorac Cardiovasc Surg. 2009; 15: 378-381.
- Yang EH, Lanks C, Shah S. Multimodality imaging of mitral perivalvular abscess with annular fistula and preserved leaflet function. Echocardiography. 2013; 30: E39-E43.