Case Report
Ann Surg Perioper Care. 2018; 3(1): 1038.
"Complicated Mural Thrombus Post-EVAR"
Arno M Wiersema1,2*, Jur K Kievit1 and Michel MPJ Reijnen3
1Vascular Surgeon, Westfriesgasthuis Hoorn, Department of Surgery, Section Vascular Surgery, Maelsonstraat 3, 1624 NP Hoorn, The Netherlands
2VrijeUniversiteit Medical Center, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands
3Vascular Surgeon, Rijnstate Ziekenhuis, Division of Vascular Surgery, Department of Surgery, Wagnerlaan 55, 6815 AD Arnhem, The Netherlands
*Corresponding author: Arno M. Wiersema, Vascular Surgeon, Department of Surgery, Section Vascular Surgery, Westfriesgasthuis, Maelsonstraat 3, 1624 NP Hoorn, The Netherlands
Received: April 12, 2018; Accepted: May 11, 2018; Published: May 18, 2018
Abstract
Purpose: To describe 4 patients with symptomatic arterial thrombo-embolic complications (ATEC) caused by mural thrombus after EVAR.
Case Report: Mural thrombus was observed in 41/123 patients (33%) in a 3-year period, four of them symptomatic (10%). Three patients suffered embolization of thrombus from a limb of the stent graft causing ischemia of the leg. In another patient mural thrombus caused a near-occlusion of the limb of the stent graft. Patients with embolic complications were treated with thrombolysis (n=1) and thrombectomy (n=2). In 3 cases the original polyester stent graft was successfully relined with a PTFE graft. In the other patient, thrombus was dislodged completely and after successful thrombolysis, no new thrombus was detected.
Conclusion: Mural thrombus formation after EVAR is a frequently encountered phenomenon. Thrombo-embolic complications are rare, but can be limb threatening. Relining the affected stent graft (after thrombolysis or thrombectomy) using a PTFE covered stent graft seems to be the best suited treatment and a durable solution.
Keywords: Abdominal aortic aneurysm; Endovascular aneurysm repair; Thrombosis; Polytetrafluoroethylene; Postoperative complications; Thrombolytic therapy
Introduction
Endovascular aortic aneurysm repair (EVAR) has become the first-choice treatment option for abdominal aortic aneurysms (AAA). Secondary interventions after EVAR remain a major concern and disadvantage of EVAR, compared to open repair of AAA [1], and represent a burden on healthcare economics [2]. One of the possible indications for secondary intervention is the development of symptomatic mural thrombus in the body of the implanted EVAR or in one of the limbs. Recently Oliveira et al. [3] published a paper on mural thrombus post-EVAR in which they observed that mural thrombus formation was a common event and present in 16,4% of 473 performed EVAR cases. They stated that the presence of mural thrombus was not associated with a higher frequency of symptomatic arterial thrombo-embolic complications (ATEC) [3]. However, other authors [4,5] have described that there is a positive correlation between the presence of mural thrombus formation and the incidence of symptomatic ATEC, including limb occlusion and distal embolization.
In this article 4 cases are described in which a mural thrombus in the EVAR graft caused symptomatic ATEC.
Case Presentation
In the period of 2012-2015 123 EVARs were performed for elective AAA repair in a single center with a minimal follow up of 6 months. Presence of mural thrombus was detected at postoperative CT in 41 patients (33%). Symptomatic ATEC was diagnosed in the 4 of them, being 3% of all EVAR and 10% of EVAR with known mural thrombus.
Case I
A 71 years old male patient presented with a 72mm infrarenal AAA. He was treated electively with an Endurant stentgraft (Medtronic, Santa Rosa, CA, USA). Anatomy was characterized by a conical shaped infrarenal aortic neck, going from 26 to 29 mm, with a length of 17mm. There was significant iliac artery angulation on both sides (85 and 92 degrees, respectively, for the left and right side). The procedure was performed without difficulties or complications under heparin prophylaxis of 5000IU. A 32-16-145 body was implanted through the right common femoral artery (CFA) in combination with a 16-24-95 extension. On the left side 2 extensions (16-16-80 and 16-24-120) were inserted. Completion angiography showed complete exclusion of the AAA with patent renal and internal iliac arteries (IIA). A minor type II endoleak was present, originating from a left latero-dorsal lumbal artery. After EVAR patient was treated with acetylsalicylic acid (ASA) 80mg and simvastatin (40mg).
A CT scan after 6 weeks confirmed adequate positioning of the stent graft with patent renal arteries and IIAs. There was no kinking of the stent graft, the type II endoleak had resolved spontaneously and no mural thrombus was present. At 12 months follow-up, duplex ultrasound (DUS) showed a new endoleak and CT scan was performed. A type II endoleak originating from a latero-dorsal lumbar artery was seen with a stable AAA diameter. A mural thrombus was observed in the left limb over a length of 36 mm and involving less than ¼ of the circumference of limb (Figure 1). No other technical or configurational abnormalities were found after careful examination. It was decided to start vitamin-K antagonists for a period of 3 months and repeat the CT scan, which showed an unchanged aspect of the mural thrombus. One month later, 18 months after initial EVAR, patient presented with an ischemic left leg with decreased motor activity, but intact sensibility (Rutherford IIb). CT scan showed that the mural thrombus of the left EVAR limb had dislodged into the femoral bifurcation where a long thrombus was seen. The superficial femoral artery (SFA) was open 2cm after its origin and a small thrombus fragment was present in the infragenual popliteal artery and at the trifurcation. Because of the clinical state it was decided to perform a surgical thrombectomy. A large thrombus was removed from the CFA, the proximal SFA and the deep femoral artery, appearing of older date. Fresh thrombus was removed from the distal SFA and popliteal artery. The post procedural course was uneventful and patient had no complaints during walking.
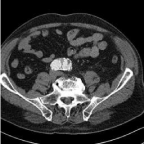
Figure 1: Mural thrombus in left limb.
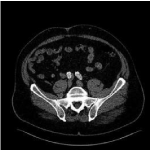
Figure 2: Thrombus on distal left limb and CIA.
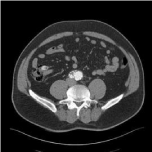
Figure 3: Near-occlusion in right limb.
After another 6 months DUS detected an increase of diameter of the AAA up to 74mm and the presence of type Ia endoleak, but no mural thrombus. Patient was treated with a proximal cuff and Endoanchors (Medtronic, Santa Rosa, CA, USA). Nine months later, a new mural thrombus was detected on CT scan at the distal end of the left EVAR limb, extending to the iliac bifurcation. Patient suffered from intermittent claudication since 1 week. Adjunctive DUS showed thrombus also being present in the SFA and proximal popliteal artery. Because of the recurrent thrombus formation inside the left limb of Endurant stent graft, it was decided to perform a thrombectomy and to reline the left limb with a 16-14, 5 polytetrafluoroethylene (PTFE) covered stent graft (Excluder, W. L. Gore & Associates, Flagstaff, AZ, USA) covering the IIA. Recovery was uneventful and patient does not suffer from (buttock) claudication until latest follow-up. CT and DUS follow up at 6 months showed a stable AAA diameter without endoleak or thrombus formation.
Case II
During a commercial total body CT scan, an infra-renal AAA of 66mm was discovered in a 67-year-old male. Anatomy showed a clock bell shaped neck of 10mm. length. A 32-16-120 Endurant body was placed during EVAR, with a 16-13-93 extension on the ipsilateral side and a 16-13-124 on the contralateral side. A cuff (32-32-45) was placed proximally to secure adequate sealing just below renal arteries. Completion angiography showed complete sealing with open renal arteries and open IAAs. No endoleak was present. During EVAR patient received 5000IU heparin intravenously and after surgery patient was put on ASA 80mg and simvastatin 40mg. No endoleak was present. CT scan at 6 weeks confirmed per-procedural findings. DUS after 6 months showed increased peak systolic velocities in body and limbs, without a significant stenosis. No mural thrombus and no endoleaks were seen. The same findings were seen 12 months later with DUS. Five months later (22 months after EVAR), patient was operated at another hospital because of an acute ischemic left leg (Rutherford II-4). Embolectomy was performed through the CFA towards proximal and distal. After successful restoration of adequate flow, a CT scan postoperatively was judged to show no abnormalities and no further treatment was warranted at that hospital. Vitamin-K antagonists were started. Only 3 months later patient was presented at our hospital with again an acute ischemic left leg. During surgical exploration a thrombus was present at the left femoral bifurcation and successful thrombectomy was performed. CT scan after surgery showed a mural thrombus still present at the distal end of the left limb and in the native CIA distal from stent (Figure 2). Regarding the fact that this thrombus most probably caused the ischemic leg, it was decided to reline the left limb of the Endurant stent graft with a PTFE covered stent graft (Gore Excluder). This was performed uneventful and a 12-10-00 graft was inserted and the IIA was over-stented intentionally. Patient experienced no (buttock) claudication and control CT and DUS at 6 and 12 months showed no abnormalities, specifically no new or old mural thrombus. Patient was treated 6 months with vitamin-K antagonists and after that with clopidogrel 75mg.
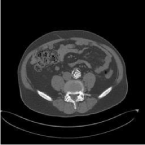
Figure 4: Limited thrombus of <25% in stent graft.
Case III
A 58-year-old male underwent x-rays for lower back pains showing a calcified 65mm AAA. CT scan confirmed this and there was strong angulation of heavily calcified iliac arteries on both sides. EVAR was performed uneventful under heparin prophylaxis of 5000 IU, using a 25-16-124 bifurcated graft (Endurant) through the left groin and extended (16-24-93). On the contra-lateral side a 16-20-156 limb was inserted. Completion angiography showed good positioning of the stent graft and a late type II endoleak, requiring no further action. CT scan after 6 weeks showed the same type II endoleak originating from a lumbar artery. According to local protocol, patient was put on ASA and simvastatin. During further follow-up until 18 months after EVAR, DUS showed a stable small type II endoleak and a stable diameter of AAA at 65 mm. DUS, 24 months after EVAR, showed a growing AAA of 73mm with turbulent flow in the right limb. CT revealed the known type II endoleak from a lumbar artery, but also an endoleak from the inferior mesenteric artery. A large mural thrombus was present in the right limb, causing a near-occlusion with diminished flow causing intermittent claudication (Figure 3). A relining was performed using 2 Gore Excluder stent grafts (20-16 and 16-16) and one Advanta V12 (Atrium Maquet, New Hampshire, USA) stent graft. It was decided to leave the type II endoleaks untreated and institute close surveillance. CT scans after 6 weeks and 6 months showed that the thrombus was completely covered by the new PTFE stent grafts and that no new intramural thrombus developed. Patient was left on ASA. Diameter of AAA stayed stable at 74mm, while the type II endoleaks remained unchanged.
Case IV
An acute EVAR for a rAAA of 72mm was performed on a 68 years old male using an aorto-uni-iliac device (Endurant 28-14-105), in conjunction with a femoro-femoral crossover bypass. Because of the rupture, no heparin was administered. Uneventful recovery followed and patient was discharged after 3 days. ASA and simvastatin were prescribed. CT scan after 6 weeks showed adequate sealing and patent renal and hypogastric arteries. No endoleak or mural thrombus was present. Follow-up until 2 years showed no abnormalities. A CT scan at 2 years showed shrinkage of the AAA to 39mm, no endoleaks but mural thrombus was present in the stent graft just above the native aortic bifurcation. Thrombus was limited to <25% of circumference and did not extend proximally or distally and a “wait and see” policy was followed (Figure 4). Nine months later, however, patient complained of a painful right leg after 150m of walking. This was present, after an acute onset, for 6 weeks. Ankle-brachial indices were 0.62 decreasing to 0.29 when walking, and DUS showed thrombus in the distal popliteal artery, slightly enlarged to 13mm, and in the crural trifurcation. Thrombolysis was performed and was successful within 48 hours. CT scan showed no popliteal artery aneurysm, but the mural thrombus from the stent graft right limb had disappeared. This thrombus likely had dislodged into popliteal artery and crural trifurcation. Patient developed a compartment-syndrome necessitating fasciotomy. The muscles could not be salvaged and 3 weeks later latissimus dorsi free muscle transplantation was done. Patient can walk now independently, with the use of a peroneus brace. No new thrombus is present on CT scan after 6 months. Patient was put on clopidogrel 75mg instead of ASA, after the thrombolysis.
Discussion
Although EVAR is the first choice treatment of AAA nowadays, it still has its limitations. The high rate of re-interventions of 20% after a follow-up period of about 4 years is a major concern [1,2,6]. Development of mural thrombus inside the stent graft is one of a less frequent complication requiring secondary intervention. The reported incidence of mural thrombus formation varies heavily between reports from 15% to 33% [3-10]. The main concern about the formation of mural thrombus is that it could lead to occlusion or distal embolization, causing an ischemic leg [4]. Contrary opinions exist if mural thrombus predisposes for limb occlusion or distal embolization [3-5,7,8,10]. In our series mural thrombus was observed in 33% of cases and eventually 10% of them became symptomatic, indicating that mural thrombus may not be harmless.
The causes of the mural thrombus formation are not completely understood. One of the possible causes is a changed hemodynamic of the blood flow after stent graft implantation [4,5,7]. The complex cascade of stent implantation, causing changed flow patterns and stent graft geometry caused by anatomic characteristics of the AAA (neck angulation, outflow through renals and IMA), are factors linked to thrombus formation [7]. An increased blood viscosity caused by smoking might also attribute of mural thrombus formation, but a recent study showed no correlation between smoking and thrombus formation after EVAR [3]. Also systemic and local factors inside the AAA and stent graft could lead to the development of post implantation syndrome [9]. The potential relation between post implantation syndrome and the formation of mural thrombus is still unclear. The presence and the amount of thrombus in the AAA before EVAR, has been suggested to play a role in development of stent graft thrombus, also because of an inflammatory reaction of the native vessel wall that is related to thrombus characteristics [5,7]. The suggestion that pressure originating from the thrombus which was present in the original AAA, influences flow patterns inside the EVAR graft, has been refuted by others [3]. Overall, no correlation was found for mural thrombus present before EVAR with stent grafts and intra-stent graft thrombus formation [3-5,7,10]
The last factor that should be considered causing mural thrombus formation after EVAR, is the type of implanted stent graft and the fabric of that graft. Maleux et al. [10] suggested that a larger diameter of the body of the stent graft increases the incidence of mural thrombus formation after EVAR. This finding was confirmed by Wu et al. [7] who demonstrated that more thrombotic deposits were present in stent grafts with a larger diameter of the main body. Also a larger difference in diameters between main body and limbs predisposes for intra-mural thrombus formation [4,7]. This could be caused by reduced flow velocity in the main body and than a “plug” flow in the tapered limbs of the stent grafts [7]. Contrary to these findings, Oliveira et al. [3] excluded the aorto-uni-iliac devices from their series of EVAR and then found no correlation between the higher ratio of cross-sectional areas of main body and limbs and the formation of mural thrombus. They did however, found that larger diameter devices and shorter necks and mid-section dilatation of the stent graft (“barrel-shape configuration”), were positively correlated to significant thrombus formation. In our series of 4 patients with complicated mural thrombus, 2 patients had a larger diameter body (32mm), but 2 not (25 and 28 mm).
When the fabric of the stent graft is related to thrombus formation, reports show that polyester is more thrombogenic than other graft materials, such as polytetrafluoroethylene (PTFE) [11,12]. Because of this much lower thrombogenicity of PTFE, we choose to reline the stent grafts with mural thrombus in our symptomatic patients with a PTFE covered stent graft, like the Gore Excluder. The polyester Endurant stent graft is our preferred graft, no higher incidence of thrombus formation has been found for the Endurant stent graft, compared to other polyester stent grafts.
During all EVAR procedures heparin is used as peri-operative antithrombotic. No data are present on the question if activated-clotting-time guided heparin administration diminishes thrombus formation post-operatively, compared to a standardized bolus of heparin (usually 5000 IU). All current guidelines on treatment of AAA advise the use of an antiplatelet agent, whether the AAA is treated conservatively or invasively. No data is available if the use of clopidogrel or acetyl salicylic acid (ASA) decreases mural thrombus formation after EVAR. The use of oral anticoagulants (vitamin K-antagonists) does not protect against mural thrombus formation. Also when they are administered after the detection of mural thrombus, no decrease in thrombus mass or protection against growth of thrombus, were seen [3,7,10].
Conclusion
Mural thrombus formation after EVAR is a frequently encountered phenomenon. Thrombo-embolic complications are rare, but can be limb threatening. Relining the affected stent graft (after thrombolysis or thrombectomy) using a PTFE covered stent graft seems to be the best suited treatment and a durable solution.
References
- De Bruin JL, Baas AF, Buth J, et al; DREAM Study Group. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010; 362: 1881-1889.
- Chambers D, Epstein D, Walker S, et al. Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model. Health Technol Assess. 2009; 13: 1-189, 215-318, iii.
- Oliveira NF, Bastos Gonçalves FM, Hoeks SE, et al. Clinical outcome and morphologic determinants of mural thrombus in abdominal aortics. J Vasc Surg. 2015; 61: 1391-1398.
- Wegener M, Gorich J, Kramer S, et al. Thrombus formation in aortic endografts. J Endovasc Ther. 2001; 8: 372-379.
- Mestres G, Maeso J, Fernandez V, et al. Incidence and evolution of mural thrombus in abdominal aortic endografts. Ann Vasc Surg. 2009; 23: 627-633.
- van Zeggeren L, Bastos Gonçalves F, van Herwaarden JA, et al. Incidence and treatment results of endurant endograft occlusion. J Vasc Surg. 2013; 57: 1246-1254; discussion 1254.
- Wu IH, Liang PC, Huang SC, et al. The significance of endograft geometry on the incidence of intraprosthetic thrombus deposits after abdominal endovascular grafting. Eur J Vasc Endovasc Surg. 2009; 38: 741-747.
- Cochennec F, Becquemin JP, Desgranges P, et al. Limb graft occlusion following EVAR: Clinical pattern, outcomes and predictive factors of occurrence. Eur J Vasc Endovasc Surg. 2007; 34: 59-65.
- Kakisis JD, Moulakakis KG, Antonopoulos CN, et al. Volume of new-onset thrombus is associated with the development of postimplantation syndrome after endovascular aneurysm repair. J Vasc Surg. 2014; 60: 1140-1145.
- Maleux G, Koolen M, Heye S, et al. Mural thrombotic deposits in abdominal aortic endografts are common and do not require additional treatment at short-term and midterm follow-up. J Vasc Interv Radiol. 2008; 19: 1558-1562.
- Hamlin GW, Rajah SM, Crow MJ, et al. Evaluation of the thrombogenic potential of three types of arterial graft studied in an artificial circulation. Br J Surg. 1978; 65: 272-276.
- Rajah SM, Crow MJ, Hamlin GW, et al. Evaluation of the thrombo-resistant properties of arterial grafts in an artificial circulation. Thromb Res. 1978; 12: 141-151.