Research Article
Ann Surg Perioper Care. 2021; 6(1): 1045.
The Effect of Biopolymer Coatings on Hernia Meshes in a Rat Model
Scheuerlein H¹* and Eisold C²
¹University of Göttingen, St. Vincenz Hospital Paderborn, Germany
²University Hospital Dresden, Medical Clinic and Polyclinic I, Germany
*Corresponding author: Hubert Scheuerlein, University of Gottingen, St. Vincenz Hospital Paderborn, Academic Teaching Hospital, Clinic for General, Visceral and Pediatric Surgery, Am Busdorf 2, 33098 Paderborn, Germany
Received: March 10, 2021; Accepted: March 29, 2021; Published: April 05, 2021
Abstract
Purpose: To improve biocompatibility and texture of hernia meshes has played a key role in tissue engineering for decades. Biopolymer (Polyethylenimine (PEI) and 3-Glycidoxypropyltrimethoxysilane (GOPS)) coating on Polypropylene (PP) and expanded Polytetrafluoroethylene (ePTFE) mesh showed promising results in fibroblast adhesion and cell growth in an invitro analysis. The objective of this animal study was to evaluate whether this may influence the incorporation into host tissue.
Methods: 30 male Lewis rats were divided into 3 groups (n=10): Group 1: ePTFE/PEI, Group 2: ePTFE/GOPS, Group 3: PP/PEI. In each animal, a 3x0.5 cm coated mesh was implanted in the right rectus sheath in sublay position, the uncoated mesh was implanted on the left equally. After 90 days, the rats were sacrificed and each side of the rectus sheath was analyzed separately for adhesions and mechanical strength. Histopathological assessment included Gieson’s stain and haematoxylin-eosin staining. The Wilcoxon test was used for statistical analyses.
Results: The GOPS-coated ePTFE tends to cause more adhesions. There was no significant difference in the mechanical strength within and between the groups, but the PEI-coated polypropylene was significantly less extendible (p<0.05) compared to the uncoated PP. In group 2, Gieson’s stain showed a significantly lower surrounding tissue reaction of foreign-body giant cells and scar tissue around the PEI-coated mesh compared to the uncoated ePTFE (p<0.05).
Conclusions: It is possible to coat surgical mesh devices with biopolymers. They do not lead to a lack of mechanical strength. The GOPS-coating did not show any general positive effect on the biocompatibility of meshes. The PEIcoating resulted in a lower surrounding tissue reaction and in a less extendible abdominal wall and should therefore be investigated further.
Keywords: Hernia repair; Mesh; Biopolymer; PEI; GOPS; Surface coatings; Biocompatibility
Introduction
Hernia repair operations, especially inguinal hernia repair procedures, are undoubtedly one of the most common procedures in general surgery. Alone about 20 millions of patients undergo inguinal hernia repair worldwide every year [1] and according to the European Hernia Society guidelines on the treatment of inguinal hernia in adult patients, elective surgery is recommended for most of the patients, although conservative management of hernia can be considered in asymptomatic cases. In the majority of the cases, mesh repairendoscopic or open is the treatment of choice as it has been shown to reduce recurrences [2]. Under day-to-day clinical conditions, commercially available meshes from materials like Polypropylene (PP), polyester and Polytetrafluoroethylene (PTFE) show good results concerning biocompatibility and mechanical strength. Hence, long-term complications like chronic pain syndromes and recurrences show that the ideal mesh has not yet been found [3,4]. The approach to improve the characteristics of these proven materials by applying different types of coatings has played a key role in tissue engineering within the past few years. Numerous additional materials such as titanium, extracellular matrix or chitosan have been tested for their effect on the occurrence of foreign body reactions and other common complications [5-7]. Polyethylenimine (PEI) and 3-Glycidoxypropyltrimethoxysilane (GOPS) are well-known and proven biopolymers in material science and technology, but have not yet been tested on hernia meshes.
PEI and GOPS
Polyethylenimine is an amine-based and alkaline biocompatible polymer. Every third atom is a tertiary amine atom, which makes it extremely cationic. PEI is already used for gene-delivery and in several in vitro studies, cells were found to proliferate faster on PEI coated surfaces [8,9].
GOPS is a functional silane and it`s epoxy is able to bind amino groups of proteins. GOPS is a proven biopolymer in dentistry, where it is used for composites [10].
In a former joint project with the chair of materials science of the Friedrich Schiller University Jena, we showed in an in vitro analysis of biopolymer (PEI and GOPS) coating on PP and expanded ePTFE mesh promising results in fibroblast adhesion and cell growth. In vitro, mouse fibroblasts grew faster and in greater numbers on the mesh surface with the PEI surface modification [11]. An increased fibroblast adhesion was observed on GOPS coated meshes as well [12,13]. The most significant results were seen with the combinations ePTFE + PEI, PP + PEI and ePTFE + GOPS. Therefore, the objective of this study was to evaluate whether these coatings may influence the incorporation into host tissue, the biocompatibility or the mechanical strength of the meshes.
Material and Methods
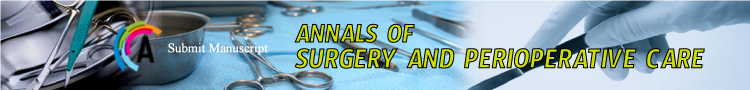
The 30 rats were divided into 3 groups (n=10) as can be seen in Table 1. In each animal, a 3x0.5 cm coated mesh was implanted in the right rectus sheath in sublay position between the rectus abdominis muscle and the peritoneum, the uncoated mesh was implanted on the left of the linea alba equally. The rats were anesthetized by 0.8 l/ min oxygen and 5% Isoflurane. Temgesic subcutaneously was used for analgesia. After disinfection with ethanol, a median skin incision of 6 cm was performed, followed by a rectus abdominis incision of 45 mm on the left and on the right of the linea alba (each in a distance of 2-3 mm to the linea alba). After the careful division of the rectus abdominis from the peritoneum, the 3x0.5 cm meshes were implanted in sublay position (Figure 1). The rectus sheath was then closed uninterruptedly with 5-0 monofilament non absorbable suture material on each side, the skin was sutured uninterruptedly with an polyfilament absorbable suture material. Ultrasound scans were performed on days 10, 30 and 90 to identify seromas and to measure the thickness of the layer between the subcutis and the mesh. After 90 days, the rats were sacrificed and each side of the rectus sheath was removed separately. Before removing the tissue samples, each side of the rectus sheath was analyzed for adhesions using a modified Vandendael score (Figure 2, [14,15]). For macroscopic evaluation a clinical score with 3 topics (tissue integration, seroma, signs of infection) according to Petter-Puchner was used [15]. The score maximum is 12 points (according to width, thickness, strength and amount, leading to grade 0-III with no, mild, moderate and severe adhesions). The compartments of rectus abdominis muscle and mesh were tested for mechanical strength using the tensile testing machine Zmart. Pro by Zwick/Roell (Institute of Textile Machinery and High Performance Material Technology, TU Dresden). The force Fmax needed to rupture the tissue sample was determined, as well as the maximum elongation of the tissue at the time Fmax. Histopathological assessment included Haematoxylin-eosin staining (Figure 3) and Van Gieson’s stain (Figure 4). In the Van Gieson’s stained specimen the thickness of the surrounding tissue reaction was measured at 5 representative points (Figure 4) and the results were correlated within the groups. The Wilcoxon test was used for statistical analyses.
Group
n
Right rectus sheath
Left rectus sheath
1
n = 10
ePTFE mesh with PEI coating
ePTFE mesh
2
n = 10
ePTFE-mesh with GOPS coating
ePTFE mesh
3
n = 10
PP mesh with PEI coating
PP mesh
Table 1: Experimental Groups.
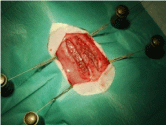
Figure 1: Mesh sublay in the left and right rectus sheath.
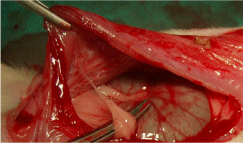
Figure 2: Left lateral perspective to both compartments with a filiform
adhesion to the left (animal 7, ePTFE/PEI).
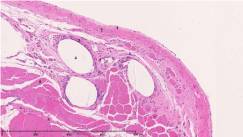
Figure 3: Haematoxylin-eosin staining:
1: Peritoneum, 2: Posterior layer of rectus sheath, 3: Surrounding tissue
reaction, 4: Mesh, 5: Rectus abdominis muscle.
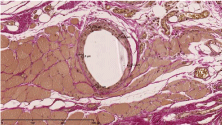
Figure 4: Van Gieson’s stain and the measurement of the thickness of the
surrounding tissue reaction.
Results
All animals survived until the end of the experiment. There was no difference in the postoperative mobility or weight gain. Seromas occurred in a total of 7 animals 4-7 days after the operation. As could be seen in the ultrasound, they were located subcutaneously underneath the cutaneous suture with no connection to the mesh compartments in all cases. The seromas disappeared within 3-9 days without any specific treatment. The GOPS-coated ePTFE tends to cause more adhesions, although the result compared to the uncoated mesh was not significant (p=0.66; altogether adhesions in 11 animals with a score of 3-5 points, in 19 animals no adhesion; p=0.317 in the ePTFE/ PEI and PP/PEI group). Macroscopic incorporation was comparable and satisfying in all groups (each animal in each group with Grade A in all 3 topics: full tissue integration, no seroma, no signs of infection). There was no significant difference in the mechanical strength within and between the groups (Figure 5), but the PEI-coated polypropylene was significantly less extendible (p<0.05) compared to the uncoated PP as can be seen in Figure 6. In the histopathological assessment (Van Gieson’s stain) group 1 (ePTFE/PEI) showed a significantly lower surrounding tissue reaction of foreign-body giant cells and scar tissue around the PEI-coated mesh compared to the uncoated ePTFE (19.84±1.85 μm vs. 22.72±4.82 μm, p<0.01). This tendency was observed in group 2 and 3 as well, although those results were not significant (19.53±4.81 μm vs. 22.88±5.89 μm, p=0.445, and 33.46±4.99 μm vs. 34.89±4.14 μm, p=0.203; Figure 7).
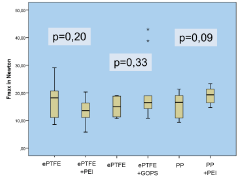
Figure 5: Mechanical strength Fmax in Newton.
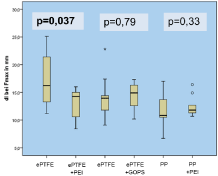
Figure 6: Extensibility of the abdominal in mm.
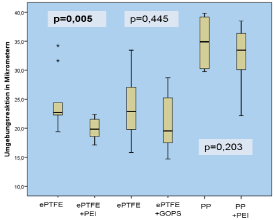
Figure 7: Surrounding tissue reaction in μm.
Discussion
Due to its clinical and financial importance, it has been the subject of research of many research groups to improve the biocompatibility of meshes by modifying the surface of commercially available materials for years. The aim of most of these investigations is to optimize the properties of established materials like PP, Polyester and ePTFE in order to reduce complications like chronic pain syndromes, adhesions and wound infections. In this study, the effect of PEI and GOPS coated meshes were investigated in an in vivo study for the first time. The incorporation into host tissue was satisfying in all groups and neither GOPS nor PEI led to a significant increased formation of adhesions. Ultrasound examinations of the abdominal wall were useful to locate and to measure seromas and showed that they were located subcutaneously without any connection to the implanted meshes. Hence, another general anaesthesia was needed for the examination. Notable was the significant lower surrounding tissue reaction of foreign-body giant cells and scar tissue around the PEI coated ePTFE meshes that could be interpreted as a sign of a better biocompatibility caused by the Polyethylenimine. The positive effect of PEI concerning the surrounding tissue reaction could be seen in the PEI coated Polypropylene as well, although these results were not significant. In general, the Polyethylenimine coating showed a stronger effect on ePTFE than on Polypropylene. This might be caused by a better surface adhesion due to the electrostatic attraction to the anionic ePTFE. Contrary to findings in the literature, both biopolymers do not lead to a lack of stability. Furthermore, the PEI coating on ePTFE leads to a significant less tensile abdominal wall. In this study, no indications could be found that GOPS has any positive effect on the properties of the meshes. Whether this is due to an insufficient adhesion of the biopolymer on the meshes or a missing effect on the fibroblasts is not clear. With special regards to a responsible use of animal experiments, only a small number of rats were included in this pilot study. Hence, the differences were small and therefore it is not possible to make any general statements about the two biopolymers, but a few informations were found to indicate that Polyethylenimine could optimize the surface of hernia meshes. The continuous further development of existing and the introduction of new materials present the surgeon with the challenge of choosing the right mesh for his patient. Therefore, promising materials should be investigated further-Polyethylenimine could be one of them.
Conclusion
It is possible to coat surgical mesh devices with biopolymers. Contrary to findings in the literature, they do not lead to a lack of mechanical strength. In our study, the GOPS-coating did not show any general positive effect on biocompatibility of meshes. The PEIcoating resulted in a lower surrounding tissue reaction and in a less extendible abdominal wall and should, therefore, be investigated further.
Many thanks to O. Dirsch (Institut für Pathologie, Klinikum Chemnitz), U. Dahmen and U. Settmacher (Universitätsklinikum Jena) and K. D. Jandt (Physikalisch-Astronomische Fakultät der Friedrich- Schiller-Universität Jena, Lehrstuhl für Materialwissenschaft) for their cooperative and beneficial support.
References
- Kulacoglu H. Current options in inguinal hernia repair in adult patients. Hippokratia. 2011; 15: 223-231.
- Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia: the journal of hernias and abdominal wall surgery. 2009; 13: 343-403.
- Bringman S, Conze J, Cuccurullo D, Deprest J, Junge K, Klosterhalfen B, et al. Hernia repair: the search for ideal meshes. Hernia: the journal of hernias and abdominal wall surgery. 2010; 14: 81-87.
- Brown CN, Finch JG. Which mesh for hernia repair? Annals of the Royal College of Surgeons of England. 2010; 92: 272-278.
- Wolf MT, Carruthers CA, Dearth CL, Crapo PM, Huber A, Burnsed OA, et al. Polypropylene surgical mesh coated with extracellular matrix mitigates the host foreign body response. Journal of biomedical materials research Part A. 2014; 102: 234-246.
- Kockerling F, Schug-Pass C. What do we know about titanized polypropylene meshes? An evidence-based review of the literature. Hernia: the journal of hernias and abdominal wall surgery. 2013.
- Udpa N, Iyer SR, Rajoria R, Breyer KE, Valentine H, Singh B, et al. Effects of chitosan coatings on polypropylene mesh for implantation in a rat abdominal wall model. Tissue engineering Part A. 2013; 19: 2713-2723.
- Lakard S, Herlem G, Propper A, Kastner A, Michel G, Valles-Villarreal N, et al. Adhesion and proliferation of cells on new polymers modified biomaterials. Bioelectrochemistry. 2004; 62: 19-27.
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995; 92: 7297-7301.
- Matinlinna JP, Choi AH, Tsoi JK. Bonding promotion of resin composite to silica-coated zirconia implant surface using a novel silane system. Clinical oral implants research. 2013; 24: 290-296.
- Scheuerlein H, Erdmann J, Rauchfuss F, Dittmar Y, Jandt K, Jandt KD, et al. [Preparation and In-Vitro Analysis of a Polyethylenimine Coating on Hernia Meshes.]. Zentralblatt fur Chirurgie. 2015.
- Erdmann J. Herstellung und In-Vitro Testung einer Polyethylenimin- Beschichtung auf Herniennetzen, Dissertation, Friedrich Schiller Universitat Jena. 2013.
- Metzler S, Zankovych S, RauchfuΒ F, Dittmar Y, Jandt K, Jandt KD, et al. In vitro analysis of biopolymer coating with glycidoxypropyltrimethoxysilane on hernia meshes. J Biomed Mater Res B Appl Biomater. 2017.
- Vandendael A, Struwig D, Nel J, Kruger T, Lombard C. Efficacy of fibrin sealant in prevention of adhesion formation on ovarian surgical wounds in a rabbit model. Gynaecological Endoscopy. 1996; 5:169-172.
- Petter-Puchner AH, Walder N, Redl H, Schwab R, Ohlinger W, Gruber-Blum S, et al. Fibrin sealant (Tissucol) enhances tissue integration of condensed polytetrafluoroethylene meshes and reduces early adhesion formation in experimental intraabdominal peritoneal onlay mesh repair. J Surg Res. 2008; 150: 190-195.